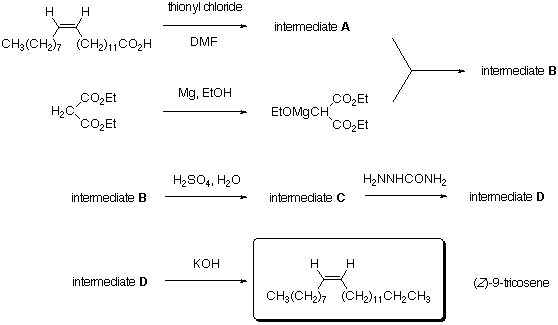
Ethoxymagnesium Diethyl Malonate. The following reaction is carried out in a 250-mL one-neck flask equipped with a heating mantle, magnetic stirrer, reflux condenser, and drying tube. A reaction mixture is prepared consisting of 1.2 g of clean magnesium turnings, 8.0 g of diethyl malonate, and 2.3 g of absolute ethanol in 25 mL of dry toluene. This mixture is heated to reflux with stirring. The reaction should begin within 5 min as indicated by a self-sustained vigorous reflux. If the reaction has not begun, 4 or 5 drops of carbon tetrachloride are added. The reaction mixture is maintained at reflux for 30-45 min, by which time most of the magnesium is consumed. This material cannot be stored effectively and is used directly.
Intermediate B. The heat is removed from the above solution of ethoxymagnesium diethyl malonate, and a solution of the intermediate A in 25 mL of dry toluene is added at once. After this reaction mixture has refluxed for 15 min, the reaction is complete, and the solvent is evaporated to afford a viscous amber residue of intermediate B.
Intermediate C. A 100-mL portion of 6 M sulfuric acid is added to the intermediate B, and the heterogeneous reaction mixture is heated at reflux. After 1 h, check the progress of the reaction by connecting the top of the reflux condenser to a piece of tubing plunging in a Ba(OH)2 solution. After 2 h, repeat this check. If the reaction has come to an end, cease refluxing. If not, continue to reflux until completion, but do not reflux for more than 3 h. After refluxing has ceased, the entire liquid contents of the flask is poured into a 125-mL separatory funnel, and the upper amber layer is separated. This material is used directly in the next step.
Intermediate D. The above crude oil is transferred in a 500-mL Erlenmeyer flask with 200 mL of methanol and 4 mL of pyridine. A 15-mL portion of 2 M aqueous semicarbazide hydrochloride is added, and the reaction mixture is heated to boiling in order to dissolve the initially formed solid and to complete the reaction. The resulting mixture is cooled to room temperature and the precipitate formed is collected by vacuum filtration and washed with 50 mL of methanol. After drying in vacuo for 30 min or overnight air drying, a pale yellow powder, m.p. 93-96 deg.C is obtained. This material is sufficiently pure for use in the next step.
(Z)-9-Tricosene. A mixture of 5 g of potassium hydroxide pellets in 50 mL of diethylene glycol is prepared in a 250-mL Erlenmeyer flask. The mixture is heated to about 225 deg.C, whereupon the potassium hydroxide dissolves and the resulting solution becomes darkly colored. A mixture of the above crude intermediate D in 25 mL of diethylene glycol is heated with swirling until dissolution is complete. The resulting solution is added at once to the hot potassium hydroxide solution. Gas evolution occurs immediately and a yellow upper layer becomes evident within 2-3 min. The reaction mixture is maintained near 225 deg.C for 20 min and then allowed to cool slowly (CAUTION: at this point you should avoid contact with the oily liquid that contains (Z)-9-tricosene, since it is readily absorbed by the skin, thereby making you attractive to flies for a long time!). The resulting dark brown mixture is poured into a separatory funnel. The upper hydrocarbon layer is separated, and the lower liquid phase is discarded.
Thin-layer chromatographic analysis of the crude product (eluent petroleum ether (b.p. 30-60 deg.C) diethyl ether 8/2 v/v) indicates one major component (Rf 0.66) and at least two relatively immobile impurities. These impurities are conveniently removed by column chromatography on 50 g of silica gel with petroleum ether (b.p. 30-60 deg.C). The elution of the pure product is monitored by TLC. Evaporation of the solvent affords 2.75 g of (Z)-9-tricosene as a colorless oil.
Sart-Tilman, le 23 octobre 1997
Nom : .............................
Prénom : ..........................
Chimie organique
Interrogation de travaux pratiques
Sur base du schéma réactionnel et du mode opératoire
ci-joints, répondez de façon brève, claire et précise aux questions suivantes.
Vos réponses doivent se trouver uniquement dans les espaces
prévus à cet effet, sur les faces recto des feuilles. Les faces
verso peuvent être utilisées comme brouillons.
Thème I. Nomenclature chimique:
1) En vous aidant du tableau ci-dessous, donnez le nom systématique de l'acide érucique (un acide gras naturel extrait du colza) (1 point)
| hydrocarbure | nom |
| C20H42 | eicosane |
| C21H44 | heneicosane |
| C22H46 | docosane |
| C23H48 | tricosane |
| C24H50 | tetracosane |
| C25H52 | pentacosane |
![]()
2) Que signifie l'abréviation DMF? Représentez sa structure (1 point)

3) Représentez la structure du chlorure de thionyle (1 point)

4) Représentez la structure du diéthylène glycol (1 point)
![]()
Thème II. Analyse du schéma réactionnel:
5) Représentez la structure de l'intermédiaire A (1 point)

6) Représentez la structure de l'intermédiaire B (1 point)

7) Représentez la structure de l'intermédiaire C (1 point)

8) Représentez la structure de l'intermédiaire D (1 point)

9) La transformation de l'intermédiaire C en (Z)-9-tricosène via l'intermédiaire D constitue une variante d'une réaction habituellement effectuée avec de l'hydrazine à la place de semicarbazide. Quel est le nom de cette réaction? (1 point)
![]()
Thème III. Analyse du mode opératoire:
III.1: Synthèse de l'intermédiaire A
10) Quel(s) est/sont le(s) gaz formé(s) au cours de la réaction? (1 point)
![]()
III.2: Synthèse de l'éthoxymagnésium malonate de diéthyle
11) Quelles pourraient être les causes du non-démarrage de la réaction? (3 points)

12) En quoi l'addition de tétrachlorure de carbone favorise-t-elle le démarrage de la réaction? (3 points)

III.3: Synthèse de l'intermédiaire C
13) Indiquez les volumes d'eau et d'acide sulfurique concentré (d = 1,84) et la façon de procéder pour préparer 100 mL de H2SO4 6 M (2 points)

14) Expliquez le rôle de la solution de Ba(OH)2 dans la détection du terme de la réaction (2 points)

III.4: Synthèse de l'intermédiaire D
15) Quelle est la structure du solide initialement formé? (1 point)

III.5: Synthèse du (Z)-9-tricosène
16) Quel(s) est/sont le(s) gaz formé(s) au cours de la réaction? (1 point)
![]()
17) Décrivez de façon détaillée, étape par étape, la façon de préparer la colonne de chromatographie et de purifier le (Z)-9-tricosène (6 points)

Question subsidiaire.
18) Imaginez un protocole expérimental simple pour mettre en évidence l'activité biologique du (Z)-9-tricosène (2 points)

Retour au sommaire des interrogations Retour au sommaire de /licence/