Synthesis of a Sedative-Hypnotic Drug: Phenobarbital

 |
Synthesis of a Sedative-Hypnotic Drug: Phenobarbital |
 |
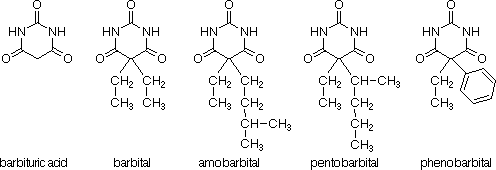
Barbiturates are central nervous system depressants used as hypnotic
drugs and anesthetics. They all derive from barbituric acid which was first
synthesized in 1864 by Adolph von Baeyer but lacked any sedative properties. At the turn of the century, Emil Fischer synthesized barbital, the first
sleep-inducing barbiturate, which was put on the market in 1903 under the name Veronal®.
Following the introduction of barbital as a drug, numerous other barbiturates
were synthesized and tested. Studies of these derivatives show that the
substituent groups at the C5 position of barbituric acid control both the
duration and the type of physiological effect that the drug induces in the
human body. Two alkyl or aryl substituents on C5 are required in order to
achieve any significant activity. Maximum sedation occurs when the alkyl chains contain five or six carbons. Subtle changes in molecular structure also affect action. Thus, amobarbital requires 30 min to take effect and sedation lasts for 5 to 6 h, while pentobarbital takes effect in 15 min but sedation lasts only for 2 or 3 h. Phenobarbital is another common drug which has been widely used in sleeping pills under trade names such as Luminal®, Gardenal®, Somonal®, etc because of its long-lasting sedative effect (up to 10 h). When the alkyl chains are getting larger, the sedative properties decrease and the substances become anticonvulsants used to treat epileptic seizures. If the alkyl groups are too long or are grafted on one of the two nitrogens of barbituric acid, convulsants are produced.
During the past decades, barbiturates were commonly administered as mild sedatives, but have since been replaced by other tranquilizers. Prolonged use of barbiturates leads to narcotic addiction. A barbiturate addict, like a heroin addict, suffers withdrawal symptoms when denied access to the drug. An overdose of barbiturates, especially when used in conjunction with the depressant alcohol, induces a deep sleep and nearly always proves fatal.
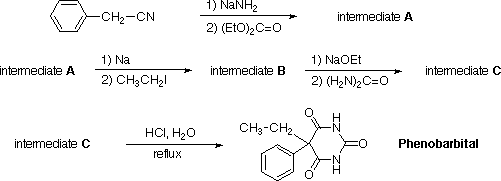
Intermediate B. To 5.8 g of clean sodium wire in anhydrous ether (150 mL), 47 g of intermediate A (0.25 mol) were added dropwise. The reaction mixture was stirred and refluxed overnight. Unreacted sodium was destroyed by adding a little methanol and fresh ether to replace loss. Freshly distilled iodoethane (50 g) was then added dropwise and the stirring and refluxing continued for 4 days. Fresh ether and more halide were added if necessary. The completion of the reaction was monitored by checking the pH of the mixture in the flask. A few drops of 20% sulfuric acid were then added followed by enough water to dissolve the precipitated solid and to cause a separation of the ether layer. The mixture was decanted and the aqueous solution extracted with fresh ether. The combined ether solutions were dried and the ether evaporated. The residue was fractionally distilled to afford 41 g of intermediate B, b.p. 147 deg.C/11 mm.
Intermediate C. Sodium ethylate, prepared from 7 g of sodium wire in 125 mL of absolute ethanol, was placed in a dry 500 mL three-necked, round-bottomed flask fitted with a reflux condenser and an addition funnel. Next, intermediate B (33 g, 0.15 mol) and urea (10 g) were added and the mixture was refluxed 8 h. The alcohol was then distilled off and the residue dissolved in about 400 mL of water. The unreacted intermediate B was removed by extraction of the water solution with ether. The aqueous solution was then acidified with a slight excess of concentrated hydrochloric acid. The white precipitate of intermediate C (12 g) was filtered off, dried, and used as crude product for the next step. If needed, pure intermediate C can be obtained by recrystallization from ethanol, m.p. 264 deg.C.
Phenobarbital. A solution of intermediate C (10 g) in 500 mL of 3 N hydrochloric acid was refluxed for a short time. Upon cooling, phenobarbital separated as a white crystalline solid, usually in a quantitative yield. Recrystallization from hot water yielded a reasonably pure material, m. p. 171 deg.C. A somewhat higher m.p. (175-176 deg.C) can be reached if ethanol is employed for recrystallization but the recovery is considerably less.
Nom : .............................
Prénom : ..........................
Sart-Tilman, le 14 octobre 1998
Sur base du schéma réactionnel et des modes opératoires ci-joints, répondez de façon brève, claire et précise aux questions suivantes.
Vos réponses doivent se trouver uniquement dans les espaces
prévus à cet effet, sur les faces recto des feuilles. Les faces
verso peuvent être utilisées comme brouillons.
Thème I. Analyse générale du mode opératoire
1) Comment procéderiez-vous pour préparer de l'éther anhydre à partir d'une bouteille de solvant pour analyse? A quel point est-il essentiel de veiller lors de cette opération? (4 points)

![]()
4) Proposez un mécanisme pour la transformation du phénylacétonitrile en intermédiaire A. Indiquez clairement sur votre schéma la structure de l'intermédiaire A. (2 points)

![]()

7) Proposez un mécanisme pour la transformation de l'intermédiaire A en B. Indiquez clairement sur votre schéma la structure de l'intermédiaire B. (2 points)


![]()
![]()
11) Proposez un mécanisme pour la transformation de l'intermédiaire B en C. Indiquez clairement sur votre schéma la structure de l'intermédiaire C. (2 points)


13) Proposez un mécanisme pour la transformation de l'intermédiaire C en phénobarbital. (2 points)


15) Que signifient les acronymes LCP et VSEPR? Proposez une traduction française de ces deux termes. (2 points)

Retour au sommaire des interrogations Retour au sommaire de /licence/