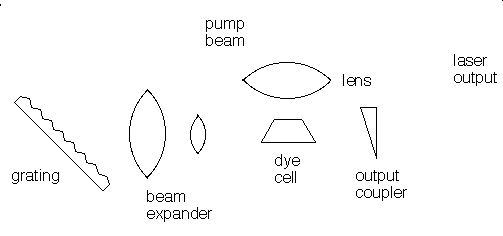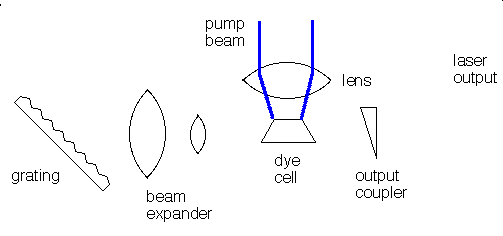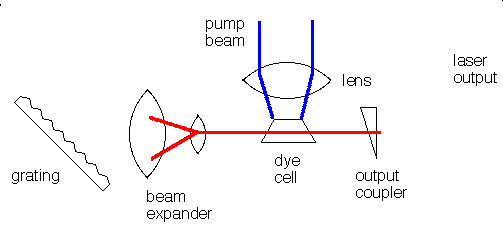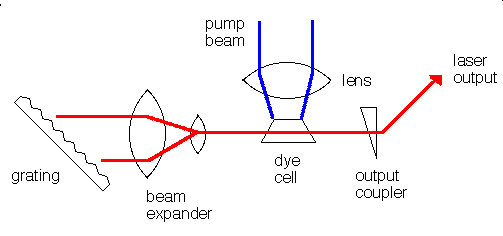
Many different materials can be used as lasers. Some, like the ruby laser, emit short pulses of laser light. Others, like helium-neon gas lasers or liquid dye lasers emit a continuous beam of light. A laser consists of at least three components:
1. a gain medium that can amplify light that passes through it
2. an energy pump source to create a population inversion in the gain medium
3. two mirrors that form a resonator cavity
The gain medium can be solid, liquid, or gas and the pump source can be an electrical discharge, a flashlamp, or another laser. The specific components of a laser vary depending on the gain medium and whether the laser is operated continuously (cw) or pulsed. In a dye laser the gain medium is an organic dye molecule that is dissolved in a solvent. The dye and solvent are circulated through a cell or a jet, and the dye molecules are excited by flashlamps or other lasers. Pulsed dye lasers use a cell and cw dye lasers typically use a jet. The organic dye molecules have broad fluorescence bands and dye lasers are typically tunable over 30 to 80 nm. Dyes exist to cover the near-UV to near-infrared spectral region: 330 - 1020 nm. para-Terphenyl is an efficient laser dye for pulsed operation because it has a high quantum efficiency and a low pump threshold. Its emission is centered at 341 nm and corresponds to the principal fluorescence peak of the molecule.

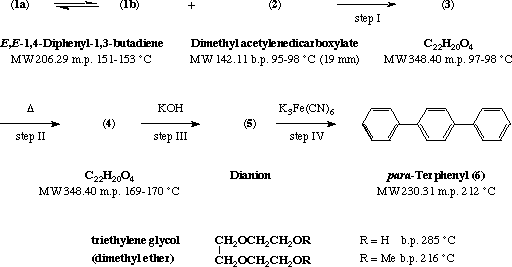
II. A solution of 2 g of 3 in 10 mL of 5% methanolic potassium hydroxide was warmed on a hot plate for about 1 min. A reddish brown color soon disappeared with separation of the isomerized product (4) as a white solid which was collected after cooling, washed with chilled methanol to remove the brown mother liquor, and dried. The yield of colorless product was 1.75 g (88%). Recrystallization from methanol gave fluorescent shiny needles, m.p. 169-170 deg.C.
III. To 1.7 g of the intermediate 4 and 0.7 g of potassium hydroxide were added 5 mL of triethylene glycol. The mixture was stirred with a thermometer and heated, raising the temperature to 140 deg.C in about 5 min. By intermittent heating, the temperature was kept close to 140 deg.C for 5 min longer before the mixture was cooled and diluted with 50 mL of water. It was heated again to boiling and in case there was a small precipitate or the solution was cloudy, pelletized activated charcoal was added and the alkaline solution of 5 was filtered by gravity.
IV. To the alkaline solution of 5 were added 3.4 g of potassium ferricyanide. The mixture was heated on a hot plate with swirling for about 5 min to dissolve the oxidant and to coagulate the white precipitate that soon separated. After filtration, the product was dried to constant weight in a vacuum oven at 100 deg.C. Recrystallization from methanol or dioxane afforded shiny flakes of para-terphenyl (6), m.p. 212 deg.C. The yield was 0.7-0.8 g (ca. 60-70% from 4).
Nom : .............................
Prénom : ..........................
Sart-Tilman, le 10 novembre 1999
Sur base du schéma réactionnel et des modes opératoires ci-joints, répondez de façon brève, claire et précise aux questions suivantes.
Vos réponses doivent se trouver uniquement dans les espaces
prévus à cet effet, sur les faces recto des feuilles. Les faces
verso peuvent être utilisées comme brouillons.
Etape I. Synthèse du composé (3)
1) Représentez la structure plane du E,E-1,4-diphényl-1,3-butadiène (1) dans ses conformations s-cis (1a) et s-trans (1b). (2 points)

![]()
![]()
Chlorure de benzyle Ph-CH2-Cl
trans-Cinnamaldéhyde Ph-CH=CH-CHO
Méthanolate de sodium CH3ONa
Triphénylphosphine Ph3P


6) Représentez les structures planes du composé (3) et de son isomère (4). (2 points)

![]()
8) Représentez la structure plane du dianion (5). (1 point)


![]()
11) Commentez la méthode de séchage du para-terphényl brut préconisée par les auteurs. Un simple séchage à l'air aurait-il pu convenir? (4 points)

Retour au sommaire des interrogations Retour au sommaire de /licence/