
Synthesis of a Sulfa Drug:
Sulfanilamide

 |
Synthesis of a Sulfa Drug: |
 |
"Sulfa drugs" is the generic name given to a group of chemotherapeutic agents derived from sulfanilamide, or para-aminobenzenesulfonamide, that are effective against a number of infectious diseases. Since sulfanilamide first came into use, more than 150 different derivatives have appeared on the market, chemically modified to achieve more effective antibacterial activity, wider spectrum of microorganisms affected, or more prolonged action. Following the discovery of penicillin, which is as effective although far less toxic, the use of sulfa drugs has somewhat declined. However, because bacteria often develop resistance to a particular kind of treatment, sulfa drugs are still used when bacterial tolerance for penicillin has developed.
The discovery of sulfa drugs was accomplished by Gerhard Domagk who had been hired in the late 1920s to head the Germany's I.G. Farben experimental pathology laboratory. Domagk performed a series of experiments on mice infected with streptococcus bacteria. He found that some previously successful compounds killed the bacteria in mice but were too toxic to give to humans. In 1935, after years of experimentation, Domagk injected an orange-red azo dye called Prontosil into a group of infected mice. The dye, which was primarily used to color wool, killed the bacteria, and, most importantly, all the mice survived. The first successful use of Prontosil on humans occurred weeks later, when Domagk gave the drug to a desperate doctor treating an infant dying of bacterial infection. The baby lived and soon did Prontosil become widely known and celebrated for its curative powers.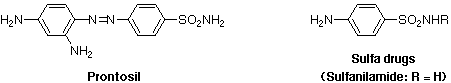
 Prontosil is an effective antibacterial substance in vivo, that
is when it is injected into a living animal. It does not display any medicinal
activity when tested in vitro, that is on a bacterial culture grown in
the laboratory. In 1935, a research group headed by Jacques
Tréfouël at the Pasteur Institute in Paris determined that
Prontosil breaks down in the body and that only a fragment of the drug molecule works against an infection. Experiments with sulfanilamide showed that it exhibited the same action as Prontosil in vivo and that it was also active in vitro, where Prontosil was inactive. It was concluded that the active portion of Prontosil was the sulfanilamide moiety. This discovery led to an explosion of interest in sulfonamide derivatives. More than 5000 different sulfa drugs were made and tested, although only a few of them ultimately proved to be of value.
Prontosil is an effective antibacterial substance in vivo, that
is when it is injected into a living animal. It does not display any medicinal
activity when tested in vitro, that is on a bacterial culture grown in
the laboratory. In 1935, a research group headed by Jacques
Tréfouël at the Pasteur Institute in Paris determined that
Prontosil breaks down in the body and that only a fragment of the drug molecule works against an infection. Experiments with sulfanilamide showed that it exhibited the same action as Prontosil in vivo and that it was also active in vitro, where Prontosil was inactive. It was concluded that the active portion of Prontosil was the sulfanilamide moiety. This discovery led to an explosion of interest in sulfonamide derivatives. More than 5000 different sulfa drugs were made and tested, although only a few of them ultimately proved to be of value.
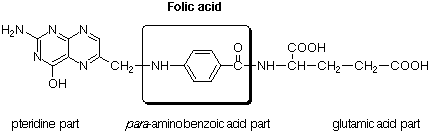
Unlike that of most other drugs, the mode of action of the sulfa drugs is now completely understood. Sulfanilamide is a competitive inhibitor for the enzymes used to synthesize folic acid, because it resembles para-aminobenzoic acid, the central chemical component of folic acid. Bacteria must synthesize folic acid in order to grow. Higher animals, like human beings, do not synthesize this vitamin, and hence must acquire it in their food. Sulfanilamide inhibits the formation of folic acid by blocking the active site of the enzyme which catalyzes the attachment of para-aminobenzoic acid to a pteridine ring system, thus effectively stopping the growth of bacteria (bacteriostatic effect). Because the synthesis of folic acid does not occur in human beings, only the bacteria are affected by the drug.
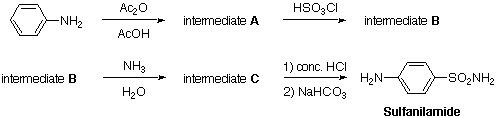
Intermediate A. Aniline (10 g) and glacial acetic acid (25 mL) were placed in a flask, followed by acetic anhydride (12 g). The mixture was briefly stirred and allowed to stand at room temperature for 5 min. It was then diluted with 100 to 200 mL of water until crystallization of the product occurred. When this was complete, the colorless lustrous crystals of intermediate A were filtered and dried under vacuum until the weight was constant. Typical yield is 70%, m.p. 113-115 deg.C.
Intermediate B. Equip a dry 250 mL two-necked round-bottomed flask with a dropping funnel and a reflux condenser. Attach the top of the latter to a device for the absorption of hydrogen chloride. Place 10 g of intermediate A (0.074 mol) in the flask and 25 mL of chlorosulfonic acid (0.385 mol) in the dropping funnel. Equip the funnel with a calcium chloride guard tube and begin the addition of chlorosulfonic acid in small portions while shaking the flask from time to time to ensure thorough mixing. When the addition is finished, heat the mixture for 1 h at 100 deg.C to complete the reaction. Allow to cool and slowly pour the oily mixture with stirring into 150 g of crushed ice contained in a 500 mL beaker. Make sure to carry out this operation very carefully in the fume cupboard. Rinse the flask with a little ice-water and add the rinsings to the content of the beaker. Break up any lumps of solid material and stir the mixture for several minutes in order to obtain an even suspension of a granular white solid. Filter off the solid and wash it with a little cold water, press and drain well. Use this crude intermediate B immediately for the next step as it tends to decompose.
Intermediate C. Transfer the crude intermediate B into the rinsed reaction flask used for its synthesis and add a mixture of concentrated ammonia solution (35 mL) and water (35 mL). Stir the content of the flask while heating to just below the boiling point for about 15 min in a fume cupboard. Cool the resulting suspension in ice and then add 6 N hydrochloric acid until the pH reaches 3. Collect the product on a Buchner funnel, wash it with a little cold water and drain as completely as possible. It is desirable but not essential to dry the crude intermediate C at 100 deg.C. The material is sufficiently pure for the next step. A small portion may be recrystallized from water with the addition of a little decolorizing carbon if necessary. The pure compound has m.p. 218 deg.C.
Sulfanilamide. Transfer the crude intermediate C to a 100 mL round-bottomed flask, add 5 mL of concentrated hydrochloric acid and 15 mL of water. Attach a reflux condenser to the flask and boil the mixture under gentle reflux for 30-45 min. Treat the cooled solution with 1 g of decolorizing carbon and filter it by gravity into a 500 mL or larger beaker. To the filtrate, cautiously add 8 g of solid sodium hydrogen carbonate in portions with stirring. Test the suspension with pH paper and if it is still acidic, add more NaHCO3 until neutral. Cool in ice, filter off the sulfanilamide with suction and dry. The yield is 7.5 g (41% overall yield), m.p. 161-163 deg.C. A pure product may be obtained by recrystallization from water or ethanol.
1. Pavia, D. L., Lampman, G. M., Kriz, G. S. Jr; Introduction to
Organic Laboratory Techniques: A Contemporary Approach, Saunders:
Philadelphia, 1976, 308-323.
2. Vogel, A. I.; Vogel's Textbook of Practical Organic Chemistry, 5th
ed., Longman: Harlow, 1989, 883-884.
3. Williamson, K. L.; Macroscale and Microscale Organic Experiments, 2nd ed., D. C. Heath: Lexington, 1994, 541-557.
Nom : .............................
Prénom : ..........................
Sart-Tilman, le 24 novembre 2000
Sur base du schéma réactionnel et des modes opératoires ci-joints, répondez de façon brève, claire et précise aux questions suivantes.
Vos réponses doivent se trouver uniquement dans les espaces
prévus à cet effet, sur les faces recto des feuilles. Les faces
verso peuvent être utilisées comme brouillons.
Etape I. Synthèse de l'intermédiaire A
1) Proposez une ou plusieurs équations moléculaires équilibrées pour rendre compte de la transformation de l'aniline en intermédiaire A dans les conditions expérimentales adoptées. Indiquez clairement sur votre schéma la structure de l'intermédiaire A. (2 points)


3) Proposez une ou plusieurs équations moléculaires équilibrées pour rendre compte de la transformation de l'intermédiaire A en B dans les conditions expérimentales adoptées. Indiquez clairement sur votre schéma la structure de l'intermédiaire B (un seul isomère). (2 points)




7) Proposez une ou plusieurs équations moléculaires équilibrées pour rendre compte de la transformation de l'intermédiaire B en C dans les conditions expérimentales adoptées. Indiquez clairement sur votre schéma la structure de l'intermédiaire C. (2 points)


9) Proposez une ou plusieurs équations moléculaires équilibrées pour rendre compte de la transformation de l'intermédiaire C en sulfanilamide dans les conditions expérimentales adoptées. (2 points)

![]()
![]()

13) Quels sont les trois types d'extincteurs disponibles au laboratoire? Comment les distingue-t-on? Pour lutter contre quel(s) type(s) de feu(x) sont-ils prévus? (3 points)


Retour au sommaire des interrogations Retour au sommaire de /licence/