Preparation of ortho-Eugenol, a Synthetic Vanilloid Fragrance
Introduction [1,2]
Eugenol and ortho-eugenol belong to a class of compounds called
vanilloids. Eugenol is a natural product extracted from the flower buds of an
evergreen tropical tree, Eugenia aromatica or clove tree, a native of
Southeast Asia. At harvest, the unopened buds are hand-picked from the tree and
sun-dried. The dry cloves contain between 14 and 20% of essential oil, the
principal component of which is eugenol and its acetyl derivative. Cloves are
strongly pungent owing to eugenol, which is extracted by steam distillation to
yield oil of cloves. Eugenol is used in germicides, perfumes, and mouthwashes,
in the synthesis of vanillin, and as a sweetener or intensifier.
ortho-Eugenol is a synthetic isomer of eugenol used in the fragrance
industry. In addition to eugenol, vanillin (from vanilla beans), ethyl vanillin
(artificial vanillin), capsaicin (the hot spice in chili peppers), and
zingerone (extracted from ginger) are other common vanilloids. All these
compounds have distinct characteristic flavors but their structures present
close similarities. They all contain a benzene ring. Subtle changes in the
sizes or positions of groups of atoms attached to the ring dramatically change
the compound's flavor, as illustrated by the following examples.
Vanillin
 |
Vanillin has a soothing, pleasant aroma. Its molecular weight is
relatively low, and it is fairly volatile. Cooking with vanilla vaporizes some
of the vanillin molecules and fills the room with its aroma. Molecules
containing only carbon and hydrogen are mostly insoluble in water. The
oxygen-containing groups attached to the ring in vanillin can form strong
hydrogen bonds with water, making it water soluble (about a gram of vanillin
can be dissolved in 100 mL of cold water). Vanillin's solubility in water is
responsible for the "finish" acquired by wines aged in oak casks. Vanilla
present in the wood lignin of the wine barrels slowly leaches into the wine
over time. |
Eugenol
|
Although eugenol is practically insoluble in water, it freely mixes with
fats and oils. Its fat solubility allows it to penetrate tissues and bind more
tightly to the vanilloid receptor, which is believed to have a fatty side
chain. The tail gives eugenol a stronger odor than vanillin has. More than one
or two ground cloves overpower a pumpkin pie. Eugenol has a numbing, analgesic
effect. It is used as a dental antiseptic (it's one component of that strange
smell some dentist's offices have). Why is the molecule an antiseptic?
Apparently the hydrocarbon tail in combination with the polar functional group
on the ring make eugenol rather soap-like, and it can disrupt the cell
membranes of bacteria the way soap disrupts a spot of grease. |  |
Zingerone
 |
Zingerone puts the zing in ginger and is also a flavor ingredient in
mustard oil. The hydrocarbon tail attached to its vanillin foundation ring
doesn't lower the solubility of zingerone much because it contains a carbonyl
group that can form strong hydrogen bonds with water molecules. Zingerone is
sparingly soluble in water, but also freely soluble in fats and oils. The
higher molecular weight of zingerone in combination with the polar side-chain
carbonyl group makes zingerone molecules attract each other more strongly than
eugenol and vanillin molecules do. As a result, zingerone is less volatile than
either eugenol or vanillin. The odor of ginger isn't strong, but the
hydrocarbon tail gives it a more intense flavor when it does come into contact
with its receptor. |
Reaction Scheme

Experimental [3]
Intermediate
In a dry 250 mL round-bottomed flask equipped with a magnetic stirring bar and
a reflux condenser fitted with a calcium chloride guard tube, introduce
guaiacol (31.1 g), 3-bromo-1-propene (31 g), anhydrous potassium carbonate
(34.6 g), and dry 2-propanol (50 mL). Reflux the mixture for 8 h then bring it
back to room temperature and add water (100 mL). Stir well until all the solids
are dissolved and transfer the biphasic mixture into a separatory funnel.
Separate the organic layer and extract twice the aqueous layer with diethyl
ether (2 x 50 mL). Gather together the 3 organic phases and wash them twice
with 10% aqueous sodium hydroxide (2 x 50 mL), then with water until pH is
neutral. Dry the etheral solution over anhydrous potassium carbonate and remove
the solvents on a rotary evaporator. Distill the residue under reduced pressure
to obtain the pure intermediate as a pale yellow oil (b.p. 117-118 deg.C/19 mm
Hg). Typical yield is 80%.
ortho-Eugenol
Place 30 g of the intermediate obtained from the previous step in a 100 mL
round-bottomed flask equipped with a reflux condenser. Heat progressively (the
reaction is slightly exothermic) until a steady reflux is achieved. Maintain
the reflux for 1 h then cool down to room temperature and dilute the reaction
mixture with diethyl ether (50 mL). Transfer the etheral solution into a
separatory funnel and extract it thrice with 10% aqueous sodium hydroxide (3 x
50 mL). Gather together the 3 aqueous phases and slowly add 18% hydrochloric
acid until pH 1. Extract thrice the acidic aqueous solution with diethyl ether
(3 x 25 mL). Gather together the 3 organic phases, dry them with anhydrous
sodium sulfate, and remove the solvents on a rotary evaporator. Distill the
residue under reduced pressure to obtain pure ortho-eugenol (b.p.
123-125 deg.C/19 mm Hg). Typical yield is 72%.
Functional Group Tests [4]
Bromine Test
Mix 5 drops of compound and 1 mL of dichloromethane into a small test tube. Add
one drop of a 2% bromine solution in dichloromethane to the tube and observe
the results. A positive test is indicated by the disappearance of the red-brown
color of the bromine.
Ferric Chloride Test
Mix 5 drops of compound and 1 mL of methanol into a small test tube. Add
one drop of neutral 1% iron(III) chloride solution in water, stopper, shake,
and observe the results. A positive test is indicated by the formation of a
highly colored solution, usually purple, blue, or green, although other colors
are possible depending upon the identity of the substituents.
Bibliography
[1] F. Senese, "Fire and Spice", In: General Chemistry Online!
(http://antoine.fsu.umd.edu/chem/senese/101/index.shtml)
[2] K. L. Williamson, Macroscale and Microscale Organic Experiments, 2nd ed., D. C. Heath: Lexington (Massachusetts), 1994, 106-107.
[3] M. Chavanne, A. Jullien, G. J. Beaudoin, E. Flamand, Chimie organique
expérimentale, Modulo: Mont-Royal (Québec), 1986, 751-756.
[4] B. S. Furniss, A. J. Hannaford, P. W. G. Smith, A. R. Tatchell, Vogel's
Textbook of Practical Organic Chemistry, 5th ed., Longman: Harlow, 1989,
1213 and 1226.
Nom : .............................
Prénom : ..........................
Sart-Tilman, le 9 novembre 2001
Seconde licence en sciences chimiques 2001-2002
Chimie organique
Interrogation de travaux pratiques
Sur base du schéma réactionnel et des modes opératoires ci-joints, répondez de façon brève, claire et précise aux questions suivantes.
Vos réponses doivent se trouver uniquement dans les espaces
prévus à cet effet, sur les faces recto des feuilles. Les faces
verso peuvent être utilisées comme brouillons.
Thème I. Nomenclature chimique:
1) Donnez le nom systématique du gaïacol. (1 point)

2) Donnez le nom usuel du 3-bromo-1-propène. (1 point)

3) Donnez le nom usuel du 2-propanol. (1 point)

Thème II. Analyse du schéma réactionnel:
4) La réaction du gaïacol avec le 3-bromo-1-propène est-elle
plus probablement de type SN1 ou SN2? Justifiez votre
réponse. (3 points)

5) L'intermédiaire se réarrange en ortho-eugénol
par sigmatropie [3,3]. Proposez un mécanisme pour cette transformation.
Indiquez clairement sur votre schéma les structures de
l'intermédiaire et de l'ortho-eugénol. La réaction
porte un nom spécifique, mentionnez-le dans votre schéma. (4
points)

Thème III. Analyse du mode opératoire:
6) Dans la synthèse de l'intermédiaire, quel est le but des
lavages avec la solution d'hydroxyde de sodium? (3 points)

7) Quelle température (approximative) faudra-t-il atteindre pour porter
le mélange réactionnel à reflux dans la synthèse de
l'ortho-eugénol? Justifiez votre réponse. (2 points)
8) Quel moyen de chauffage disponible au laboratoire utiliseriez-vous pour
atteindre et maintenir le reflux discuté à la question
précédente. Justifiez votre réponse. (2 points)

9) Quelle source de vide disponible au laboratoire utiliseriez-vous pour
effectuer la distillation de l'intermédiaire et de
l'ortho-eugénol? Justifiez votre réponse. (2 points)

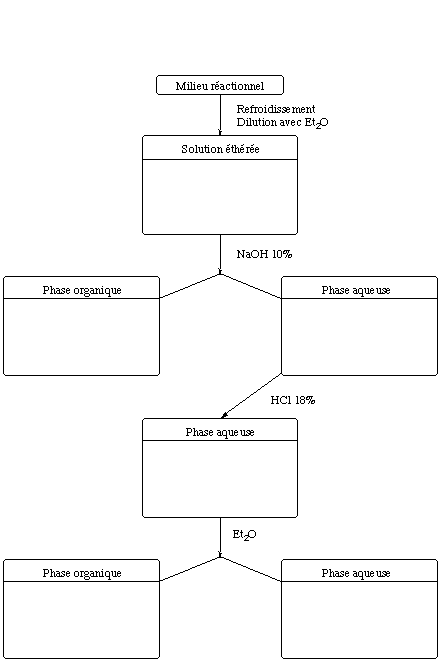
10) Dans la synthèse de l'ortho-eugénol, quel est le but
des extractions avec la solution d'hydroxyde de sodium? Pour étayer
votre réponse, complétez le diagramme suivant en indiquant la
composition en matières organiques et inorganiques des diverses phases
(4 points)
Thème IV. Tests de caractérisation:
11) Quel(s) groupement(s) fonctionnel(s) le test au brome permet-il de
détecter? Justifiez votre réponse. (2 points)

12) Proposez un réactif inorganique permettant de détruire le
brome. Écrivez l'équation moléculaire
équilibrée de la réaction. (2 points)

13) Quel(s) groupement(s) fonctionnel(s) le test au chlorure ferrique permet-il
de détecter? Justifiez votre réponse. (2 points)

14) Complétez le tableau suivant en indiquant par un signe + les tests
qui donneront un résultat positif et par un signe - ceux qui donneront
un résultat négatif. (1 point)

Retour au sommaire des interrogations Retour au sommaire de /licence/


![]()
![]()
![]()










