
Preparation of trans-Stilbene Under Phase Transfer Conditions

 |
Preparation of trans-Stilbene Under Phase Transfer Conditions |
 |
Laundry detergents which promise a "dazzling white" or "livelier colors" usually contain fluorescent whitening agents (FWAs), also known as fluorescent brightening agents or optical brighteners to do the trick. As clothes get older, they reflect yellow light which makes them look faded. Whitening agents in detergents make them look new and fresh by absorbing ultraviolet light (from the sun or from fluorescent bulbs) and by emitting a bluish light to make colored clothes appear brighter and white ones "whiter than white".
Fluorescent whiteners were originally introduced in the textile manufacture because natural fibers such as cotton and wool, as well as synthetic fibers such as polyester and polyamide (nylon) have an inherent yellowish color that cannot be removed through chemical bleaching processes. FWAs are therefore required to produce the color white. Nearly all printing papers and whitelined paperboards also contain fluorescent whitening agents. In 1992, the world consumption of FWAs was estimated at 60 000 tons. Fifty percent was consumed by the detergent industry, 33% by the paper industry, and 17% by the textile industry.
Chemically speaking, a FWA is a fluorescent molecule, i.e. it absorbs light in a certain region of the wavelength spectrum (typically 340-360 nm) and emits it in a different one (mainly 430 to 460 nm). As a consequence of fluorescence blue light is added on a (normally yellow) substrate, thereby increasing the perceived whiteness. A FWA is characterized by its absorption and fluorescence maximum, its quantum yield, and its affinity for a substrate.
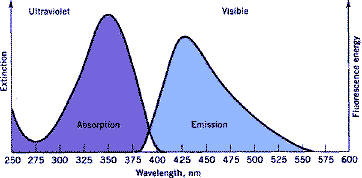
Optical brightening started by observing that washing bleached cotton with certain types of chestnut imparted washed goods a whiter appearance. Research showed that the compound responsible for this activity was a natural coumarin derived from esculetin that showed a blue fluorescence (structure 1). Since then, many fluorescent compounds have been investigated to provide a suitable whitening effect. Only a small number of them have found practical use, however, and doubtless stilbene derivatives are the ones with the highest application volumes.
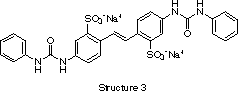
Today, most commercial brighteners are derivatives of 4,4'-diaminostilben-2,2'-disulfonic acid (structure 2). The usual compounds are symmetric. Generally, they are highly conjugated molecules having a planar structure and anionic charges. Asymmetric derivatives can also be synthesized, however their preparation is more expensive and they show little advantage over their symmetrical counterparts. The principal effect of structural variations are changes in solubility, substrate affinity, acid fastness, etc. For instance, the phenylureido derivative known as Blankophor® R (structure 3) was patented by Bayer for dyeing cellulose, nylon, wool, or paper.
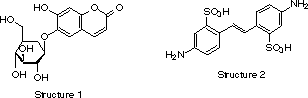
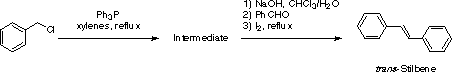
Intermediate3,4
Charge a 250 mL round-bottom flask with 10 g of benzyl chloride, 100 mL of xylenes and 21.6 g of triphenylphosphine. Equip the flask with an upright condenser and reflux the mixture for 18 h. After cooling down to room temperature, filter the white crystalline precipitate and rinse it twice with 10 mL of diethyl ether. Recrystallization from chloroform/petroleum ether (b.p. 60-80 deg.C) affords the pure intermediate as colorless needles (m.p. 338-339 deg.C). Typical yield is 70%.
trans-Stilbene5,6
1. In a 250 mL plastic beaker, dissolve 50 g of sodium hydroxide into 75 mL of cold distilled water and wait until the solution comes back to room temperature.
2. Meanwhile, add 10 mL of benzaldehyde to 30 mL of a 10% aqueous sodium carbonate solution contained in a 100 mL Erlenmeyer flask. Shake vigorously the biphasic mixture for a few minutes, then allow the two layers to separate again.
3. In a 250 mL Erlenmeyer flask containing a magnetic stirring bar, prepare a suspension of the intermediate (7.78 g, 20 mmol) in 15 mL of dichloromethane. Carefully remove 2 mL of benzaldehyde (20 mmol) from the first Erlenmeyer flask with a pipette (it forms the top layer) and add it to the intermediate in CH2Cl2. Start stirring the organic mixture and slowly add the sodium hydroxide solution. Keep under vigorous stirring for 30 minutes then transfer the mixture into a separatory funnel and rinse the reaction flask with dichloromethane (2 x 20 mL) and water (15 mL). Separate the organic phase (due to the density of the aqueous base used, dichloromethane should constitute the upper layer - check before discarding any of the phases). Dry the dichloromethane solution over magnesium sulfate and remove the solvent on a rotary evaporator. Triturate the residue repeatedly with hot petroleum ether (b.p. 40-60 deg.C, 4 x 25 mL) and combine the supernatant solutions. Discard the remaining solid.
4. Add a crystal of iodine to the petroleum ether solution and reflux the mixture for 30 minutes to effect isomerization. Cool down to room temperature and decolorize the mixture with 10 mL of a 5% aqueous sodium metabisulfite solution. Transfer the biphasic mixture into a separatory funnel and remove the lower aqueous phase, then add methanol (25 mL) to the remaining organic phase. If two phases do not form, add water dropwise until this occurs. Separate the upper layer of petroleum ether, dry it over magnesium sulfate and remove the solvent on a rotary evaporator. Purify the solid by sublimation to obtain crystals of pure trans-stilbene (m.p. 124 deg.C). Typical yield is 60%.
1. "Fluorescent Whitening Agents", In Kirk-Othmer Encyclopedia of Chemical Technology 4th ed, J. E. Kroschwitz (Ed.), Wiley-Interscience: New York, 1994, vol. 11, pp. 227-241.
2. "Les azurants optiques", S. Lemeune, http://www.geocities.com/Paris/LeftBank/5810/fwa.html
3. J. Grimshaw, J. S. Ramsey, J. Chem. Soc. B 1968, 63-64.
4. P. Warner, R. Sutherland, J. Org. Chem. 1992, 57, 6294-6300.
5. G. Märkl, A. Merz, Synthesis 1973, 295-297.
6. L. M. Harwood, C. J. Moody, Experimental Organic Chemistry, Blackwell: Oxford, 1989, pp. 586-588.
Nom : .............................
Prénom : ..........................
Sart-Tilman, le 15 novembre 2002
Sur base du schéma réactionnel et des modes opératoires ci-joints, répondez de façon brève, claire et précise aux questions suivantes.
Vos réponses doivent se trouver uniquement dans les espaces
prévus à cet effet, sur les faces recto des feuilles. Les faces
verso peuvent être utilisées comme brouillons.
Toutes vos équations chimiques doivent être complètes et équilibrées. Soignez les dessins de structures chimiques et de montages expérimentaux.
Thème I. Analyse du schéma réactionnel:
1) Donnez le nom et la structure de l'intermédiaire. (1 point)



5) L'intermédiaire est préparé dans les xylènes. Indiquez la nature des molécules ou classes de molécules qui constituent ce solvant. (2 points)

![]()
7) (Paragraphe 1) Pourquoi la solution d'hydroxyde de sodium est-elle préparée dans un bécher en plastique plutôt qu'en verre? (1 point)






Donnée: en présence d'eau, le métabisulfite de sodium se transforme en hydrogénosulfite de sodium selon la réaction: Na2S2O5 + H2O -> 2 NaHSO3


Retour au sommaire des interrogations Retour au sommaire de /licence/