 | Enantioselective synthesis of ibuprofen, a non-steroidal anti-inflammatory drug |
Ibuprofen (2-(4-isobutylphenyl)propanoic acid) is an effective non-steroidal analgesic and anti-inflammatory agent. As such, it is one of the top-ten drugs sold worldwide under various trade names including Advil®, Nurofen®, Motrin®, Nuprin®, and Brufen®. While uses may vary in different countries, ibuprofen is generally indicated for the relief of mild to moderate pain, for the reduction in fever, and for reduction of inflammation. A range of more specific therapeutic recommendations also exists. In the UK for instance, it is licensed for use in headaches, colds and flu, rheumatic pain, muscular pain, backache, feverishness, migraine, period pain, dental pain, and neuralgia.1,2 |  |
The patent to the propionic acid derivatives of which ibuprofen is a member was filed in 1964 by a British team from the Boots company lead by Dr Stewart Adams.3 In 1949, when this research started, only two pain relieving anti-inflammatory drugs were available for treating rheumatoid arthritis: aspirin and cortisone. The objectives of the research were to develop a drug, which had a superior therapeutic profile and less adverse side effects than these two drugs. The discovery of ibuprofen achieved both of these objectives.4
Ibuprofen, like all non-steroidal anti-inflammatory agents acts via the inhibition of the enzyme cyclooxygenase (COX), which in turn reduces the level of prostaglandins in the body. Prostaglandins are chemical mediators involved in pain sensation, inflammation, and fever and ibuprofen is effective in reducing all three of these effects. Unlike aspirin, ibuprofen has a relatively weak antithrombotic effect, being a weak inhibitor of platelet aggregation. Good evidence exists to suggest that it is safer on the stomach than aspirin, having a safety profile similar to that of paracetamol. It is also more potent than aspirin or paracetamol, 400 mg of ibuprofen being roughly equivalent in effect to 1000 mg of aspirin or paracetamol.1
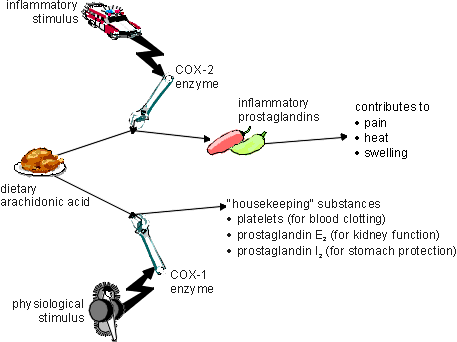
While commercial ibuprofen is currently marketed as a racemic mixture, it has been shown that only the S enantiomer has the desired biological activity. Slow enzymatic racemization of the chiral center ensures that much of the ingested drug is eventually converted into the active form. Yet, while racemic ibuprofen requires almost 40 minutes to take effect, the same dosage of the S enantiomer needs no more than a third of that time. For this reason, as well as to avoid potential deleterious side effects of the R enantiomer, pharmaceutical companies have become increasingly interested in producing the enantiomerically pure drug.
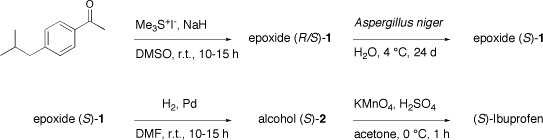
1. Synthesis of Epoxide (RS)-1
Under a nitrogen atmosphere, sodium hydride (4.1 g, 60% dispersion in mineral oil, ca. 103 mmol) was carefully added to a solution of trimethylsulfonium iodide (20.8 g, 102 mmol) in 200 mL of DMSO. The solution was stirred for 15 min at room temperature, then 4-isobutylacetophenone (15 g, 85 mmol) dissolved in 50 mL of DMSO was added dropwise over a 20 min period of time. The reaction mixture was stirred overnight. It was poured into 1 L of water and the crude product was extracted with 2 x 200 mL of pentane. The pentane fractions were gathered, washed with 2 x 300 mL of water, dried over MgSO4, and concentrated in vacuum. The oily residue was distilled under reduced pressure to afford pure epoxide (RS)-1 as a colorless oil (b.p. 95 °C / 4 mm Hg) in 87% yield.
2. Enzymatic Resolution of Epoxide (RS)-1
Aspergillus niger enzymatic extract (2.6 g) was dissolved in 30 mL of 0.4 M aqueous Tris/Tris.HCl at 4 °C (pH = 8.0). To this solution was added 1.5 g of epoxide (RS)-1. The mixture was shaken for 24 days in a cold room at 4 °C. The reaction was stopped by extraction of the epoxide (S)-1 with 3 x 10 mL of pentane. The pentane fractions were concentrated in vacuum and used without any further purification for the next step.
3. Synthesis of Alcohol (S)-2
A suspension of palladium powder (0.2 g, 2 mmol) in 20 mL of DMF was stirred for 20 min in a hydrogen atmosphere at room temperature. The mixture was cooled to 0 °C and the crude epoxide (S)-1 obtained from the previous step was added. The reaction mixture was stirred overnight at 0 °C under hydrogen. It was then poured into 150 mL of water. The aqueous phase was extracted with 2 x 35 mL of pentane. The pentane fractions were dried over MgSO4 and concentrated in vacuum. The residue was purified by flash chromatography on silica gel with pentane/dichloromethane 3/1 (v/v) as eluant. The alcohol (S)-2 was obtained as a colorless oil in 35% yield and 95% ee starting from the racemic epoxide (RS)-1.
4. Synthesis of (S)-Ibuprofen
Alcohol (S)-2 (2.76 mmol) was dissolved in 12 mL of 3M H2SO4 in acetone. The mixture was cooled in an ice/water bath with stirring before potassium permanganate (0.79 g, 5 mmol) was added portionwise at 0 °C. The mixture was kept for 1 h at 0 °C, the cooling bath was then removed and stirring was prolonged for 2 more hours at room temperature. Solid sodium hydrogen sulfite was added until the solution became colorless. The resulting mixture was diluted with 50 mL of water and extracted with 2 x 25 mL of ether/pentane 1/2 (v/v). The organic phases were gathered and extracted twice with 2 x 50 mL of a 2% aqueous NaOH solution. The aqueous phases were gathered and brought to pH 1 with concentrated HCl. The turbid acidic mixture was extracted with 2 x 50 mL of chloroform. The organic phases were gathered, dried over MgSO4, and concentrated in vacuum. The residue was purified by flash chromatography on silica gel with dichloromethane/ether 4/1 (v/v) as eluant. (S)-Ibuprofen was isolated as a sticky solid in 76% yield, m.p. 48 °C.
A small amount of the product was treated with thionyl chloride, followed by (S)-a-methylbenzylamine and subjected to HPLC analysis. Optical purity was 95%.
1 Ibuprofen, http://www.disprin.com/students/main_ibu.htm.
2 J. Bower, Ibuprofen http://www.chm.bris.ac.uk/motm/ibuprofen/homepage.htm.
3 J. S. Nicholson, S. S. Adams, Brit. Pat. 971700 (1964); Chem. Abstr. 61, 14591d (1964).
4 S. S. Adams, Chem. Brit. 1193-1195 (1987).
5 S. E. Sen, K. S. Anliker, J. Chem. Educ. 73, 569-572 (1996).
6 M. Cleij, A. Archelas, R. Furstoss, J. Org. Chem. 64, 5029-5035 (1999).
Nom : .............................
Prénom : ..........................
Sart-Tilman, le 22 novembre 2004
Sur base du schéma réactionnel et des modes opératoires ci-joints, répondez de façon brève, claire et précise aux questions suivantes. Vos réponses doivent se trouver uniquement dans les espaces prévus à cet effet, sur les faces recto des feuilles. Les faces verso peuvent être utilisées comme brouillons. Toutes vos équations chimiques doivent être complètes et équilibrées. Soignez les dessins de structures chimiques et de montages expérimentaux.
Thème I. Synthèse de l'époxyde (RS)-1
1) Proposez un schéma de synthèse alternatif en deux étapes faisant intervenir une réaction de Wittig suivie d'une époxydation par mCPBA pour transformer la 4-isobutylacétophénone en époxyde (RS)-1 si l'on ne dispose pas d'iodure de triméthylsulfonium au laboratoire. Indiquez clairement sur votre schéma la structure de l'époxyde (RS)-1. (2 points)

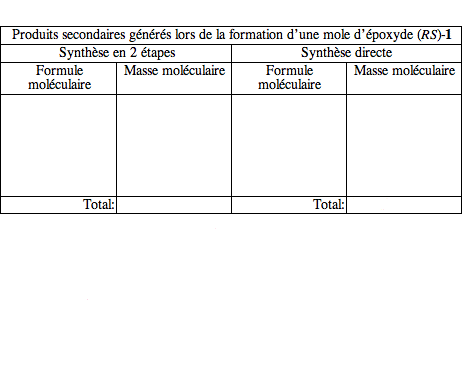
2) En complétant et en commentant le tableau ci-dessous, comparez le schéma de synthèse décrit à la question 1 et la réaction directe effectuée au moyen d'iodure de triméthylsulfonium en termes d'économie d'atomes et d'élimination des déchets. (3 points)
3) Représentez schématiquement le montage expérimental que vous utiliseriez pour distiller l'époxyde (RS)-1. Discutez plus particulièrement la façon dont vous procéderiez pour maintenir une pression de 4 mm Hg. (3 points)

Thème II. Résolution enzymatique de l'époxyde (RS)-1
4) Représentez la structure de l'époxyde (S)-1 en indiquant clairement sa stéréochimie et la façon dont vous l'avez déterminée. (2 points)

5) Le tris(hydroxyméthyl)aminométhane et son hydrochlorure (Tris et Tris.HCl) sont couramment utilisés en biochimie pour maintenir le pH dans la zone physiologique (pH = 7-8). Expliquez succinctement comment ils assurent cette fonction. (2 points)

Thème III. Synthèse de l'alcool (S)-2
6) Proposez une équation moléculaire équilibrée rendant compte de la transformation de l'époxyde (S)-1 en alcool (S)-2. Indiquez clairement sur votre schéma la structure de l'alcool (S)-2 et sa stéréochimie. (2 points)

7) En quoi une chromatographie flash diffère-t-elle d'une chromatographie liquide habituelle? Quels sont les avantages et inconvénients des deux techniques? (2 points)

8) Que signifie l'abréviation v/v? Illustrez votre réponse en prenant comme exemple le mélange éluant décrit dans le mode opératoire pour la chromatographie flash de l'alcool (S)-2. (2 points)

Thème IV. Synthèse du (S)-ibuprofen
9) Proposez une équation moléculaire équilibrée rendant compte de la transformation de l'alcool (S)-2 en (S)-ibuprofen dans les conditions expérimentales adoptées. Indiquez clairement sur votre schéma la structure du (S)-ibuprofen et sa stéréochimie. (2 points)

10) Au moyen d'une équation moléculaire équilibrée, expliquez comment l'hydrogénosulfite de sodium provoque une décoloration du milieu réactionnel. Quelle était selon vous la coloration initiale? (2 points)

11) A quoi servent les différents lavages et extractions effectués lors de la purification du (S)-ibuprofen? (2 points)

12) Que signifie l'acronyme HPLC? Expliquez de manière succincte le fonctionnement d'un appareil de HPLC en annotant le schéma ci-après. (3 points)
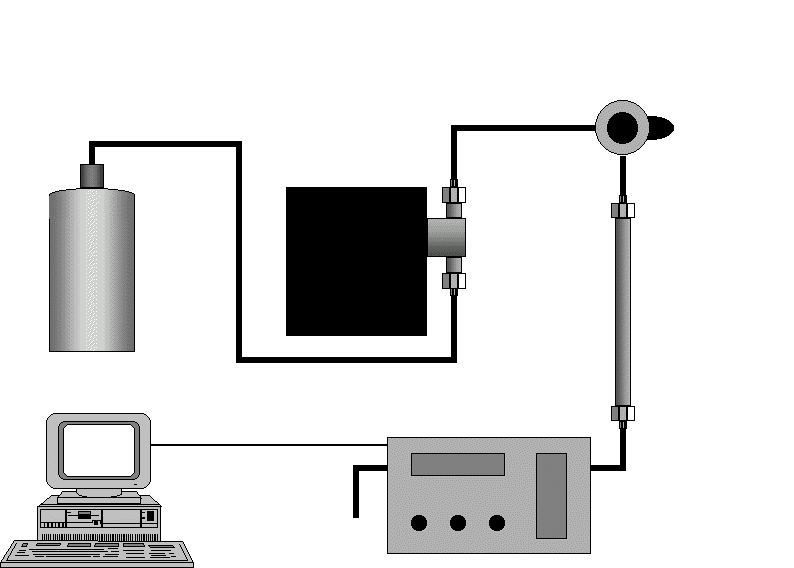
13) Au moyen d'une ou plusieurs équations moléculaires équilibrées, décrivez le traitement appliqué au (S)-ibuprofen avant son analyse par HPLC. A quoi sert ce traitement? (3 points)

Retour au sommaire des interrogations Retour au sommaire de /licence/