"New In situ Generated Ruthenium Catalysts Bearing N-Heterocyclic Carbene Ligands for the Ring-Opening Metathesis Polymerization of Cyclooctene"
Lionel Delaude, Magdalena Szypas, Albert Demonceau, and Alfred F. Noels
 |
source: Advanced Synthesis & Catalysis
year: 2002
volume: 344
first page: 749
last page: 756
doi: 10.1002/1615-4169(200208)344:6/7<749::AID-ADSC749>3.0.CO;2-T
|
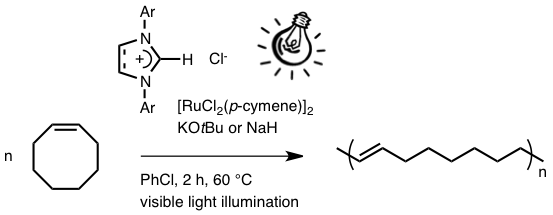
Abstract: New 1,3-diarylimidazol(in)ium chlorides bearing phenyl, 1-naphtyl, 4-biphenyl, 2-tolyl, 2,6-dimethylphenyl, and 3,5-dimethylphenyl substituents were synthesized. They were combined with [RuCl2(p-cymene)]2 and potassium tert-butoxide or sodium hydride to generate the corresponding ruthenium-N-heterocyclic carbene complexes in situ. Catalyst precursors derived from imidazol(in)ium salts bearing the 2,4,6-trimethylphenyl (mesityl) and the 2,6-diisopropylphenyl groups were also prepared. The catalytic activity of all these species in the photoinduced ring-opening metathesis polymerization of cyclooctene was investigated. The C4-C5 double bond in the imidazole ring of the N-heterocyclic carbene ligands was not crucial to achieve high catalytic efficiencies. The presence or the absence of alkyl groups on the ortho positions of the phenyl rings had a more pronounced influence. Blocking all the ortho positions was a requisite for obtaining efficient catalysts. Failure to do so probably results in the ortho-metallation of the carbene ligand, thereby altering the coordination sphere of the ruthenium active centers.
Keywords: N-Heterocyclic Carbene (NHC) ligands, Homogeneous Catalysis, Metathesis, Polymerization, Ring-Opening Metathesis Polymerization (ROMP); Ruthenium
[Full Text] [<< Previous Article] [Back to the List of Publications] [Next Article >>] l.delaude@ulg.ac.be