"Stereoselective Synthesis of (E)-Hydroxystilbenoids by Ruthenium-Catalyzed Cross-Metathesis"
Karine Ferré-Filmon, Lionel Delaude, Albert Demonceau, and Alfred F. Noels
 |
source: European Journal of Organic Chemistry
year: 2005
first page: 3319
last page: 3325
doi: 10.1002/ejoc.200500068
|
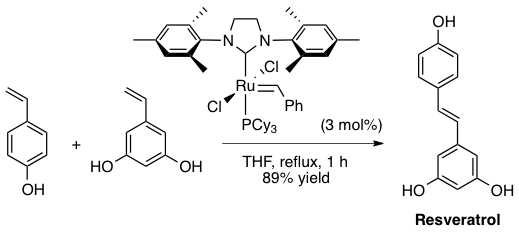
Abstract: An efficient and highly stereoselective synthetic procedure is reported for the construction of symmetrical and unsymmetrical (E)-polymethoxystilbene and (E)-polyhydroxystilbene derivatives. The strategy rests on a cross-metathesis reaction catalyzed by stable, well-defined (alkylidene)ruthenium complexes, in particular the second-generation Grubbs catalyst [RuCl2(=CHPh)(SIMes)(PCy3)] [SIMes = 1,3-bis(2,4,6-trimethylphenyl)imidazolidin-2-ylidene]. The metathesis of unprotected phenolic styrenes is illustrated by the synthesis of the important phytoalexins (E)-3,4',5-trihydroxystilbene (resveratrol) and (E)-3,3',4,5'-tetrahydroxystilbene (piceatannol).
Keywords: Alkenes, Homogeneous Catalysis, Natural Products, Resveratrol, Ruthenium
[Full Text] [<< Previous Article] [Back to the List of Publications] [Next Article >>] l.delaude@ulg.ac.be