"Facile Microwave-Assisted Synthesis of Cyclic Amidinium Salts"
Adila Aidouni, Soufiane Bendahou, Albert Demonceau, and Lionel Delaude
 |
source: Journal of Combinatorial Chemistry
year: 2008
volume: 10
first page: 886
last page: 892
doi: 10.1021/cc800101k
|
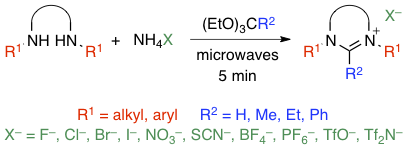
Abstract: The cyclization of N,N'-dialkyl or diaryl ethane-1,2-diamines or propane-1,3-diamines with inorganic ammonium salts and orthoesters proceeds briskly under microwave irradiation to afford the corresponding imidazolinium or tetrahydropyrimidinium salts. The transformation is highly versatile and tolerates a wide range of substituents and counterions. It could be scaled up from 1 to 50 mmol without any difficulty. Because the workup is equally rapid and straightforward, this experimental procedure provides a fast and convenient access to an important class of heterocyclic compounds that have found numerous applications as N-heterocyclic carbene precursors, organocatalysts and ionic liquids.
[Full Text] [<< Previous Article] [Back to the List of Publications] [Next Article >>] l.delaude@ulg.ac.be