"Betaine Adducts of N-Heterocyclic Carbenes: Synthesis, Properties, and Reactivity"
Lionel Delaude
 |
source: European Journal of Inorganic Chemistry
year: 2009
first page: 1681
last page: 1699
doi: 10.1002/ejic.200801227
|
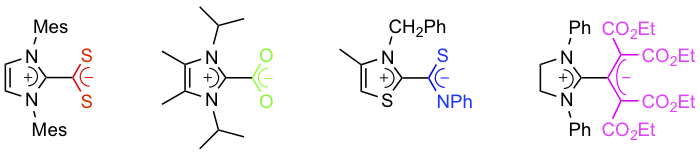
Abstract: N-Heterocyclic carbenes (NHCs) form stable zwitterionic adducts with a range of heteroallenes, ketenes, and allenes. Although the first representatives of this class of inner salts were first investigated as far back as the 1960s, they have enjoyed a sustained interest from the chemical community over the years. Depending on the nature of their anionic moiety, NHC betaines display a very broad palette of reactivities and have found applications in various fields of organic synthesis and catalysis. In this Microreview, the synthesis, properties, and reactivity of NHC betaines are surveyed. The NHCs under consideration include ylidenes derived from imidazole, benzimidazole, imidazoline, thiazole, or triazole, and the heteroallenes investigated so far are carbon dioxide, carbon disulfide, isocyanates, isothiocyanates, and their selenium analogues. A historical background is provided for each type of adduct under consideration, but emphasis is placed mainly on developments that have appeared in the literature within the past few years.
Keywords: Betaines, Carbene Ligands, N-heterocyclic Carbenes, Homogeneous Catalysis, Nitrogen Heterocycles, Zwitterions
[Full Text] [<< Previous Article] [Back to the List of Publications] [Next Article >>] l.delaude@ulg.ac.be