"Synthesis and Catalytic Evaluation of Ruthenium-Arene Complexes Generated Using Imidazol(in)ium-2-carboxylates and Dithiocarboxylates"
Lionel Delaude, Xavier Sauvage, Albert Demonceau, and Johan Wouters
 |
source: Organometallics
year: 2009
volume: 28
first page: 4056
last page: 4064
doi: 10.1021/om9002363
|
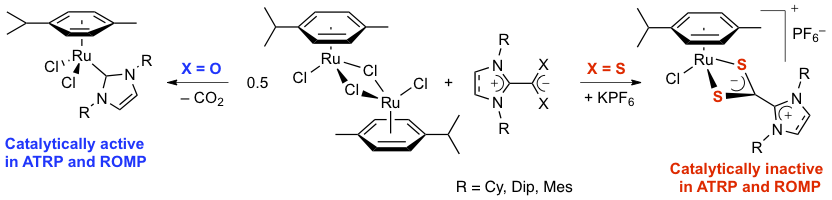
Abstract: The ability of five imidazol(in)ium-2-carboxylates and dithiocarboxylates bearing cyclohexyl, mesityl, or 2,6-diisopropylphenyl substituents on their nitrogen atoms to act as NHC precursors for in situ catalytic applications was probed in ruthenium-promoted ring-opening metathesis and atom transfer radical polymerizations. Results obtained with 1:2 mixtures of [RuCl2(p-cymene)]2 and NHC · CO2 adducts were in line with those reported previously starting from preformed [RuCl2(p-cymene)(NHC)] complexes, whereas the NHC · CS2 zwitterions were almost completely inactive. To account for this dichotomy, the preparation of preformed ruthenium-arene complexes from [RuCl2(p-cymene)]2 and NHC · CX2 inner salts was thoroughly investigated. As expected, imidazolium-2-carboxylates lost their CO2 moiety and afforded [RuCl2(p-cymene)(NHC)] complexes in high yields, whereas the NHC · CS2 betaines retained their zwitterionic nature and led to cationic complexes of the [RuCl(p-cymene)(NHC · CS2)]PF6 type. These stable, 18-electron species are the first examples of well-defined transition-metal complexes bearing chelating NHC · CS2 ligands. They were characterized by various analytical techniques, and the molecular structure of [RuCl(p-cymene)(IMes · CS2)]PF6 was determined by X-ray diffraction analysis.
[Full Text] [<< Previous Article] [Back to the List of Publications] [Next Article >>] l.delaude@ulg.ac.be