"Mixed Isobutylphobane/N-Heterocyclic Carbene Ruthenium-Indenylidene Complexes: Synthesis and Catalytic Evaluation in Olefin Metathesis Reactions"
Xavier Sauvage, Guillermo Zaragoza, Albert Demonceau, and Lionel Delaude
 |
source: Advanced Synthesis & Catalysis
year: 2010
volume: 352
first page: 1934
last page: 1948
doi: 10.1002/adsc.201000207
|
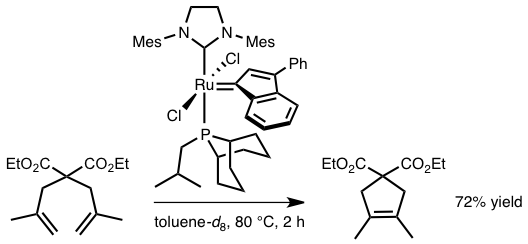
Abstract: Two new second generation ruthenium(II) dichloride-indenylidene complexes [RuCl2(9-isobutylphosphabicyclo[3.3.1]-nonane)(NHC)(3-phenyl-1-indenylidene)], where NHC = 1,3-bis(2,4,6-trimethylphenyl)imidazolin-2-ylidene (SIMes) or its unsaturated imidazol-2-ylidene analogue (IMes), were isolated in high yields upon heating a tetrahydrofuran (THF) solution of the diphosphane complex [RuCl2(isobutylphobane)2(3-phenyl-1-indenylidene)] with a two-fold excess of the corresponding imidazol(in)ium-2-carboxylate zwitterions. Both products were characterized by 1H, 13C, and 31P NMR spectroscopy, and the molecular structure of [RuCl2(isobutylphobane)(SIMes)(3-phenyl-1-indenylidene)] was determined by X-ray diffraction analysis. A close inspection of the packing structure revealed the presence of different types of intra- and intermolecular interactions that enhanced the global stability of the crystals, while low temperature NMR experiments showed the existence of two distinct rotational isomers due to the unsymmetrical nature of the phobane ligand. The catalytic activity of both compounds was assessed in olefin metathesis using benchmark ring-opening metathesis polymerization, ring-closing metathesis (RCM), and cross-metathesis reactions, and compared with those of related first and second generation ruthenium-benzylidene and indenylidene catalyst precursors. Kinetic studies confirmed the high thermal stability of the mixed isobutylphobane/N-heterocyclic carbene complexes, which suffered from a slow initiation efficiency compared to other catalytic systems based on the tricyclohexylphosphane ligand. However, the remarkable robustness of [RuCl2(isobutylphobane)(SIMes)(3-phenyl-1-indenylidene)] was beneficial for performing the RCM of diethyl 2,2-bis(2-methylallyl)malonate. Monitoring the formation of the ruthenium-methylidene active species [RuCl2(isobutylphobane)-(SIMes)(=CH2)] derived from this precursor further demonstrated its ability to sustain long reaction times and high temperatures required to carry out the RCM of tetrasubstituted olefins.
Keywords: Ethenolysis, Homogeneous Catalysis, N-Heterocyclic Carbenes, Phosphane Ligands, Ring-Closing Metathesis, Ruthenium
[Full Text] [<< Previous Article] [Back to the List of Publications] [Next Article >>] l.delaude@ulg.ac.be