"Synthesis and Catalytic Evaluation in Olefin Metathesis of a Second-Generation Homobimetallic Ruthenium-Arene Complex Bearing a Vinylidene Ligand"
Yannick Borguet, Xavier Sauvage, Guillermo Zaragoza, Albert Demonceau, and Lionel Delaude
 |
source: Organometallics
year: 2011
volume: 30
first page: 2730
last page: 2738
doi: 10.1021/om2001074
|
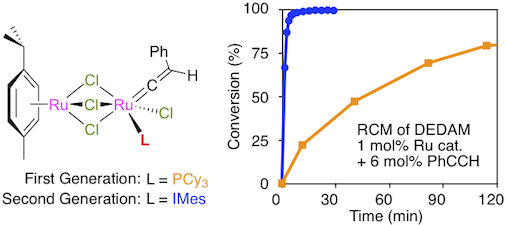
Abstract: The new homobimetallic ruthenium-vinylidene complex [(p-cymene)Ru(μ-Cl)3RuCl(=C=CHPh)(IMes)] (6) was isolated in high yield upon treatment of [(p-cymene)Ru(μ-Cl)3RuCl(η2-C2H4)(IMes)] (5) with a slight excess of phenylacetylene at -50 °C. Although it was very stable under normal atmosphere in the solid state, this product underwent an oxidative cleavage into the corresponding carbonyl compound [(p-cymene)Ru(μ-Cl)3RuCl(CO)(IMes)] (7) when dissolved in oxygen-containing solvents. Second-generation complexes 6 and 7 were characterized by IR and NMR spectroscopies, and their molecular structures were determined by X-ray diffraction analysis. The catalytic activity of complex 6 was probed in various types of olefin metathesis reactions. Compared to its first-generation analogue, the new ruthenium initiator displayed an enhanced activity. It was also much more selective than ruthenium-ethylene complex 5. Aluminum chloride was a valuable cocatalyst for the ROMP of cyclooctene, while phenylacetylene was better suited to achieve the fast and quantitative RCM of α,ω-dienes into the corresponding di- or trisubstituted cycloolefins. The role of the terminal alkyne was rationalized by assuming that it would allow an enyne metathesis to take place initially, thereby transforming saturated vinylidene precursor 6 into a highly active mono- or bimetallic ruthenium-alkylidene species.
[Full Text] [<< Previous Article] [Back to the List of Publications] [Next Article >>] l.delaude@ulg.ac.be