"Continuous-Flow N-Heterocyclic Carbene Generation and Organocatalysis"
Lorenzo Di Marco, Morgan Hans, Lionel Delaude, and Jean-Christophe M. Monbaliu
 |
source: Chemistry - A European Journal
year: 2016
volume: 22
first page: 4508
last page: 4514
doi: 10.1002/chem.201505135
|
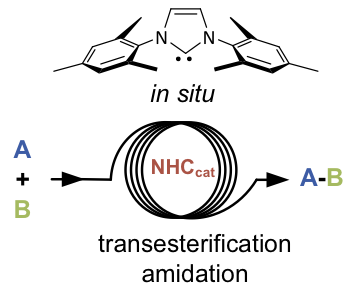
Abstract: Two methods were assessed for the generation of common N-heterocyclic carbenes (NHCs) from stable imidazol(in)ium precursors using convenient and straightforward continuous-flow setups with either a heterogeneous inorganic base (Cs2CO3 or K3PO4) or a homogeneous organic base (KN(SiMe3)2). In-line quenching with carbon disulfide revealed that the homogeneous strategy was most efficient for the preparation of a small library of NHCs. The generation of free nucleophilic carbenes was next telescoped with two benchmark NHC-catalyzed reactions; namely, the transesterification of vinyl acetate with benzyl alcohol and the amidation of N-Boc-glycine methyl ester with ethanolamine. Both organocatalytic transformations proceeded with total conversion and excellent yields were achieved after extraction, showcasing the first examples of continuous-flow organocatalysis with NHCs.
Keywords: Carbenes, Continuous-Flow, Microreactors, Organocatalysis, Transesterification
[Full Text] [<< Previous Article] [Back to the List of Publications] [Next Article >>] l.delaude@ulg.ac.be