"Synthesis of Azolium-2-dithiocarboxylate Zwitterions Under Mild, Aerobic Conditions"
François Mazars, Madalina Hrubaru, Nikolay Tumanov, Johan Wouters, and Lionel Delaude
 |
source: European Journal of Organic Chemistry
year: 2021
first page: 2025
last page: 2033
doi: 10.1002/ejoc.202100274
|
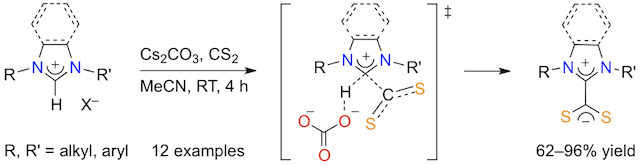
Abstract: Twelve imidazolium-, imidazolinium-, or benzimidazolium-2-dithiocarboxylate zwitterions with aliphatic or aromatic substituents on their nitrogen atoms, including four new unsymmetrical 1-alkyl-3-arylimidazolium derivatives, were obtained in high yields (62-96%) upon reaction of azolium salts with CS2 and Cs2CO3 in acetonitrile at room temperature. Compared to the previous strategies devised for the synthesis of NHC·CS2 betaines, this novel procedure relied on an innocuous, weak base and could be applied under mild aerobic conditions. All the new compounds were fully characterized by various analytical techniques and the molecular structures of two of them were determined by XRD analysis. An associative mechanism involving the concerted reaction of the azolium salts with both CS2 and CO32- was tentatively proposed to account for the formation of the zwitterionic adducts without the intervention of free carbenes. This would explain the good results obtained with a weak inorganic base that lacks the strength needed to deprotonate the azolium salt substrates.
Keywords: Carbenes, Nitrogen Heterocycles, Reaction Mechanisms, S Ligands, Synthetic Methods
[Full Text] [<< Previous Article] [Back to the List of Publications] [Next Article >>] l.delaude@ulg.ac.be