"Ruthenium Complexes Bearing Zwitterionic Imidazolium-dithiocarboxylate Ligands Derived from N-Heterocyclic Olefins"
François Mazars, Guillermo Zaragoza, Johann Far, and Lionel Delaude
 |
source: Submitted for publication
year: 2026
volume:
first page:
last page:
doi:
|
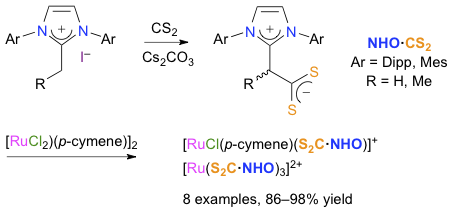
Abstract: C2-alkylated imidazolium salts reacted with carbon disulfide and cesium carbonate under mild, aerobic conditions to afford imidazolium-dithiocarboxylate zwitterions. The addition of CS2 took place regioselectively on the exocyclic C2α position of the starting materials and four new compounds were isolated in satisfactory to high yields. Two of them featured a chiral center and were obtained as racemic mixtures. Compared to the previous strategies that required a strong base to deprotonate the imidazolium salts, followed by the nucleophilic addition of the intermediate N-heterocyclic olefins (NHOs) onto carbon disulfide, our method involved only one step and relied on a weak, innocuous base. Moreover, it did not necessitate to use dry and degassed solvents under an inert atmosphere, making it very environmental-friendly and easy to implement. The four NHO·CS2 zwitterions served as ligands to prepare a small library of heteroleptic ruthenium-arene complexes with the generic formula [RuCl(p-cymene)(S2C·NHO)](PF6) and homoleptic dicationic complexes of the type [Ru(S2C·NHO)3](PF6)2. These chelates are the first examples of coordination compounds based on NHO·CS2 inner salts. They were fully characterized using various analytical techniques and the molecular structures of three of them were determined. We also synthesized the IMes=CH2·CS2-d2 betaine in three steps from 1,3-dimesitylimidazolium chloride (IMes·HCl) using a straighforward procedure, and we used it as an isotopically labeled ligand to form the stable [RuCl(p-cymene)(S2C·CH2=IMes-d2)](PF6) complex in 95% yield. This experiment served as a proof of concept to demonstrate the potentials of our methodology for the design of new metallodrugs. Lastly, the catalytic activity of the eight Ru(S2C·NHO) chelates was probed in the transfer hydrogenation of acetophenone with isopropanol and potassium hydroxide. Very gratifyingly, they proved superior to their Ru(S2C·NHC) cousins featuring xanthinium-8-dithiocarboxylate ligands derived from caffeine and theophylline.
[Full Text] [<< Previous Article] [Back to the List of Publications] [Next Article >>] l.delaude@ulg.ac.be