"A Novel Oxidizing Reagent Based on Potassium Ferrate (VI)"
Lionel Delaude and Pierre Laszlo
 |
source: Journal of Organic Chemistry
year: 1996
volume: 61
first page: 6360
last page: 6370
doi: 10.1021/jo960633p
|
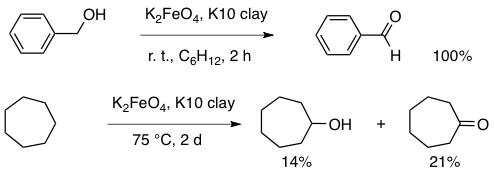
Abstract: A new, efficient preparation has been devised for potassium ferrate(VI) (K2FeO4). The ability of this high-valent iron salt for oxidizing organic substrates in nonaqueous media was studied. Using benzyl alcohol as a model, the catalytic activity of a wide range of microporous adsorbents was ascertained. Among numerous solid supports of the aluminosilicate type, the K10 montmorillonite clay was found to be best at achieving quantitative formation of benzaldehyde, without any overoxidation to benzoic acid. The roles of the various parameters (reaction time and temperature, nature of the solvent, method of preparation of the solid reagent) were investigated. The evidence points to a polar reaction mechanism. The ensuing procedure was applied successfully, at room temperature, to oxidation of a series of alcohols to aldehydes and ketones, to oxidative coupling of thiols to disulfides, and to oxidation of nitrogen derivatives. At 75 °C, the reagent has the capability of oxidizing both activated and nonactivated hydrocarbons. Toluene is turned into benzyl alcohol (and benzaldehyde). Cycloalkanes are also oxidized, in significant (30-40%) yields, to the respective cycloalkanols (and cycloalkanones). Thus, potassium ferrate, used in conjunction with an appropriate heterogeneous catalyst, is a strong and environmentally friendly oxidant.
[Full Text] [<< Previous Article] [Back to the List of Publications] [Next Article >>] l.delaude@ulg.ac.be