"Synthesis of Stilbenoids via the Suzuki-Miyaura Reaction Catalysed by Palladium N-Heterocyclic Carbene Complexes"
Adriana Tudose, Anna M. Maj, Xavier Sauvage, Lionel Delaude, Albert Demonceau, and Alfred F. Noels
 |
source: Journal of Molecular Catalysis A:Chemical
year: 2006
volume: 257
first page: 158
last page: 166
doi: 10.1016/j.molcata.2006.04.064
|
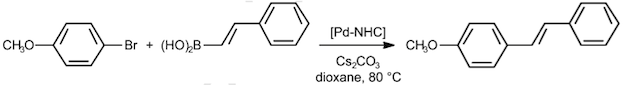
Abstract: The Suzuki-Miyaura reaction of aryl halides with trans-2-phenylvinylboronic acid using a series of related in situ generated N-heterocyclic carbene palladium(II) complexes was studied in order to evaluate the effect of ligand structure and electronics on the catalytic activity and to investigate the nature of the catalyst species. The nature of the substituents of the carbene ligand was found to be critical. Specifically, the presence of alkyl groups on the ortho positions of the phenyl substituents was a requisite for obtaining the most efficient catalyst systems.
Keywords: Aryl Halides, Boronic Acids, Homogeneous Catalysis, N-Heterocyclic Carbene Ligands, Palladium and Compounds, Stilbenes, Suzuki-Miyaura Coupling Reactions
[Full Text] [<< Previous Article] [Back to the List of Publications] [Next Article >>] l.delaude@ulg.ac.be