"Imidazol(in)ium-2-carboxylates as N-Heterocyclic Carbene Ligand Precursors for Suzuki-Miyaura Reactions"
Adriana Tudose, Lionel Delaude, Benoît André, and Albert Demonceau
 |
source: Tetrahedron Letters
year: 2006
volume: 47
first page: 8529
last page: 8533
doi: 10.1016/j.tetlet.2006.09.139
|
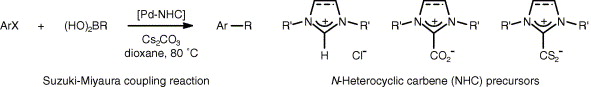
Abstract: Simple catalysts formed in situ from palladium acetate and a variety of imidazolium and imidazolinium carboxylates and dithiocarboxylates have been screened in the coupling of aryl halides with trans-2-phenylvinylboronic acid. Imidazol(in)ium carboxylates show an excellent activity, which compares to that displayed by the parent imidazol(in)ium chlorides, whereas imidazol(in)ium dithiocarboxylates are poorly efficient. Interestingly, the base employed exerts a profound influence on the trans/cis stereochemistry of the coupling product.
Keywords: Aryl Halides, Boronic Acids, Homogeneous Catalysis, N-Heterocyclic Carbene Ligands, Palladium and Compounds, Stilbenes, Suzuki-Miyaura Coupling Reactions
[Full Text] [<< Previous Article] [Back to the List of Publications] [Next Article >>] l.delaude@ulg.ac.be