"Synthesis of Biphenylamines via Suzuki-Miyaura Cross-Coupling Reactions"
Anna M. Maj, Lionel Delaude, Albert Demonceau, and Alfred F. Noels
 |
source: Tetrahedron
year: 2007
volume: 63
first page: 2657
last page: 2663
doi: 10.1016/j.tet.2007.01.023
|
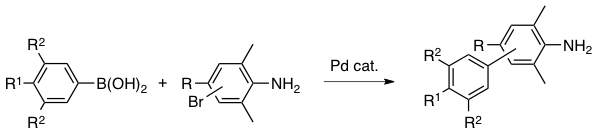
Abstract: A small library of meta- and para-biphenylamines substituted by various alkyl, alkoxy, phenoxy, or halogeno groups on their aromatic rings was synthesized via Suzuki-Miyaura cross-coupling between bromoanilines and arylboronic acids using palladium catalysts. The experimental conditions were carefully adjusted to accommodate a wide range of substituents, in terms of electron-withdrawing or -donating ability and steric bulk. In some cases, protection and deprotection of the amine function via its trifluoroacetamide were added to the reaction sequence in order to facilitate the cross-coupling step.
Keywords: Amines, Biaryls, Cross-Coupling, Homogeneous Catalysis, Palladium
[Full Text] [<< Previous Article] [Back to the List of Publications] [Next Article >>] l.delaude@ulg.ac.be