"Synthesis of N-Heterocyclic Carbene Precursors Bearing Biphenyl Units and Their Use in Ruthenium-Catalyzed Ring-Opening Metathesis Polymerization"
Anna M. Maj, Lionel Delaude, Albert Demonceau, and Alfred F. Noels
 |
source: Journal of Organometallic Chemistry
year: 2007
volume: 692
first page: 3048
last page: 3056
doi: 10.1016/j.jorganchem.2007.03.027
|
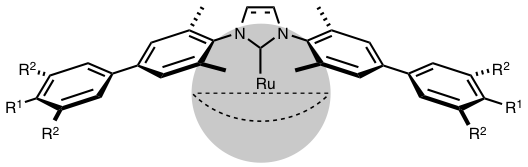
Abstract: A range of new imidazolium and imidazolinium chlorides bearing biphenyl units on their nitrogen atoms was synthesized. They differed by the electron-withdrawing or -donating nature and the steric bulk of the substituents on their aromatic rings. These various N-heterocyclic carbene (NHC) precursors were combined with the [RuCl2(p-cymene)]2 dimer and potassium tert-butoxide to generate the corresponding ruthenium-arene complexes [RuCl2(p-cymene)(NHC)] in situ. The catalytic activity of these species was investigated in the photoinduced ring-opening metathesis polymerization (ROMP) of cyclooctene. The results obtained confirmed the necessity of blocking the ortho-positions of the phenyl rings in the vicinity of the metal center in order to attain high catalytic efficiencies. They also showed that changing the steric and electronic properties of the substituents on the remote phenyl rings of the biphenyl units had no significant influence on the outcome of the polymerization.
Keywords: Arene Ligands, Biaryls, Carbene Ligands, Cyclooctene, Homogeneous Catalysis, Substituent Effects
[Full Text] [<< Previous Article] [Back to the List of Publications] [Next Article >>] l.delaude@ulg.ac.be