"Ruthenium-Arene Complexes Bearing Imidazol(in)ium-2-dithiocarboxylate Ligands: Evaluation of Their Catalytic Activity in the Synthesis of Enol Esters"
Quentin Willem, François Nicks, Xavier Sauvage, Lionel Delaude, and Albert Demonceau
 |
source: Journal of Organometallic Chemistry
year: 2009
volume: 694
first page: 4049
last page: 4055
doi: 10.1016/j.jorganchem.2009.08.028
|
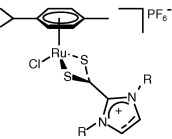
Abstract: The catalytic activity of four ruthenium imidazol(in)ium-2-dithiocarboxylates was evaluated for the synthesis of vinyl esters through addition of 4-acetoxybenzoic acid to 1-hexyne, and compared to those of the parent ruthenium-N-heterocyclic carbene complexes and [RuCl2(p-cymene)(PPh3)] (a standard catalyst). It turned out that ruthenium imidazol(in)ium-2-dithiocarboxylates were poorly active and selective. Quantitative yields, indeed, were obtained only after extended reaction times. However, the catalytic activity could be improved significantly under microwave heating or conventional heating in a sealed tube at 160 °C, driving the reaction to completion in less than 4 h of reaction.
Keywords: Alkynes, Carboxylic Acids, Enol Esters, N-Heterocyclic Carbene Ligands, Microwave, Ruthenium
[Full Text] [<< Previous Article] [Back to the List of Publications] [Next Article >>] l.delaude@ulg.ac.be