"Microwave-Assisted Synthesis of 1,3-Dimesitylimidazolinium Chloride"
Morgan Hans and Lionel Delaude
 |
source: Organic Syntheses
year: 2010
volume: 87
first page: 77
last page: 87
doi: not available
|
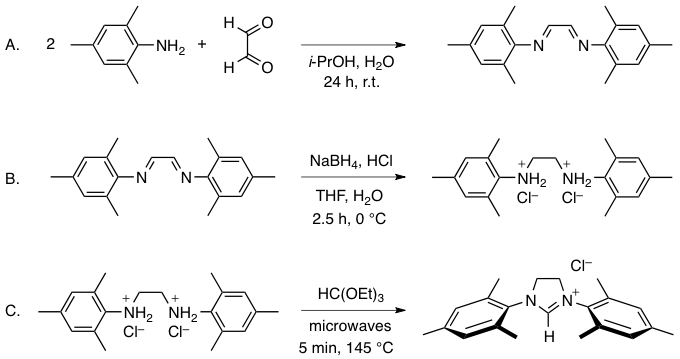
Abstract: A procedure for the microwave-assisted synthesis of 1,3-dimesitylimidazolinium chloride on a preparative scale is described starting from simple, commercially available reagents. Prior to a microwave-assisted cyclization, it involves the formation of N,N'-dimesitylethane-1,2-diamine dihydrochloride via condensation of glyoxal with two equivalents of mesitylamine, followed by reduction of the intermediate Schiff base with sodium borohydride under acidic conditions. All three steps proceed readily under normal atmosphere. Laboratory grade solvents and reagents taken straight from the bottles do not require any additional purification. The two intermediates and the final product are isolated in high yield and purity by simple filtration and washing and may be used without any further purification for most applications.
[Full Text] [<< Previous Article] [Back to the List of Publications] [Next Article >>] l.delaude@ulg.ac.be