"Homobimetallic Ruthenium-Arene Complexes Bearing Vinylidene Ligands: Synthesis, Characterization, and Catalytic Application in Olefin Metathesis"
Yannick Borguet, Xavier Sauvage, Guillermo Zaragoza, Albert Demonceau, and Lionel Delaude
 |
source: Organometallics
year: 2010
volume: 29
first page: 6675
last page: 6686
doi: 10.1021/om1006177
|
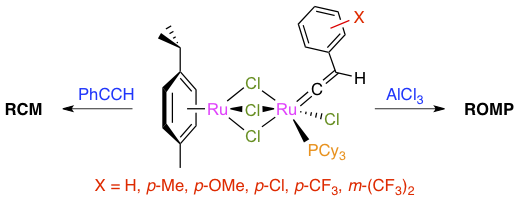
Abstract: Five new arylvinylidene complexes with substituents ranging from electron-donating to strongly withdrawing (p-OMe, p-Me, p-Cl, p-CF3, and m-(CF3)2) were isolated in high yields by reacting [(p-cymene)Ru(μ-Cl)3RuCl(η2-C2H4)(PCy3)] (3) with the corresponding phenylacetylene derivatives. The known phenylvinylidene complex [(p-cymene)Ru(μ-Cl)3RuCl(=C=CHPh)(PCy3)] (5) was also obtained from [RuCl2(p-cymene)]2, tricyclohexylphosphine, and phenylacetylene under microwave irradiation. The influence of the remote aryl substituents on structural features was investigated by IR, NMR, and XRD spectroscopies. A very good linear relationship was observed between the chemical shift of the vinylidene α-carbon atom and the Hammett σ-constants of the aryl group substituents. The catalytic activity of the six homobimetallic complexes was probed in various types of olefin metathesis reactions. Unsubstituted phenylvinylidene compound 5 served as a lead structure for these experiments. Its reaction with norbornene afforded high molecular weight polymers with a broad polydispersity index and mostly trans double bonds. Aluminum chloride was a suitable cocatalyst for the ring-opening metathesis polymerization of cyclooctene and led to the formation of high molecular weight polyoctenamer with a rather narrow polydispersity index (Mw/Mn = 1.25) and an almost equimolar proportion of cis and trans double bonds. No major changes were observed in the polymer yields and microstructures when complexes bearing donor groups on their aryl rings were employed as catalyst precursors. On the other hand, compounds bearing strongly electron-withdrawing substituents were significantly less active. Model vinylidene compound 5 and its ruthenium-ethylene parent (3) both required the addition of phenylacetylene to achieve the ring-closing metathesis of diethyl 2,2-diallylmalonate. Thus, the role of this terminal alkyne cocatalyst goes beyond the facile replacement of the η2-alkene ligand with a vinylidene fragment.
[Full Text] [<< Previous Article] [Back to the List of Publications] [Next Article >>] l.delaude@ulg.ac.be