"Tandem Catalysis of Ring-Closing Metathesis/Atom Transfer Radical Reactions with Homobimetallic Ruthenium-Arene Complexes"
Yannick Borguet, Xavier Sauvage, Guillermo Zaragoza, Albert Demonceau, and Lionel Delaude
 |
source: Beilstein Journal of Organic Chemistry
year: 2010
volume: 6
first page: 1167
last page: 1173
doi: 10.3762/bjoc.6.133
|
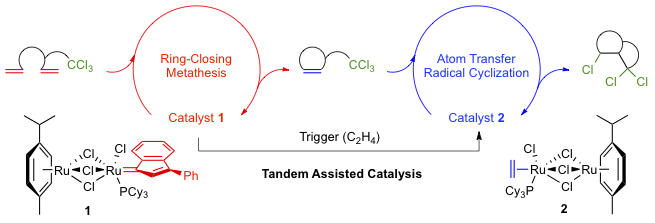
Abstract: The tandem catalysis of ring-closing metathesis/atom transfer radical reactions was investigated with the homobimetallic ruthenium-indenylidene complex [(p-cymene)Ru(μ-Cl)3RuCl(3-phenyl-1-indenylidene)(PCy3)] (1) to generate active species in situ. The two catalytic processes were first carried out independently in a case study before the whole sequence was optimized and applied to the synthesis of several polyhalogenated bicyclic γ-lactams and lactones from α,ω-diene substrates bearing trihaloacetamide or trichloroacetate functionalities. The individual steps were carefully monitored by 1H and 31P NMR spectroscopies in order to understand the intimate details of the catalytic cycles. Polyhalogenated substrates and the ethylene released upon metathesis induced the clean transformation of catalyst precursor 1 into the Ru(II)-Ru(III) mixed-valence compound [(p-cymene)Ru(μ-Cl)3RuCl2(PCy3)], which was found to be an efficient promoter for atom transfer radical reactions under the adopted experimental conditions.
Keywords: Grubbs Catalyst, Indenylidene Ligands, Kharasch Reaction, Microwave Heating, Olefin Metathesis
[Full Text] [<< Previous Article] [Back to the List of Publications] [Next Article >>] l.delaude@ulg.ac.be