"Synthesis and Catalytic Evaluation of Ruthenium-Arene Complexes Bearing Imidazol(in)ium-2-thiocarboxylate Ligands"
Morgan Hans, Quentin Willem, Johan Wouters, Albert Demonceau, and Lionel Delaude
 |
source: Organometallics
year: 2011
volume: 30
first page: 6133
last page: 6142
doi: 10.1021/om2006529
|
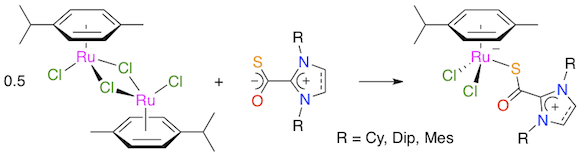
Abstract: Five new complexes with the generic formula [RuCl2(p-cymene)(SOC·NHC)] (2-6) were isolated in high yields by reacting the [RuCl2(p-cymene)]2 dimer with a range of imidazol(in)ium-2-thiocarboxylate zwitterions bearing cyclohexyl, 2,4,6-trimethylphenyl (mesityl), or 2,6-diisopropylphenyl groups on their nitrogen atoms in CH2Cl2 at -20 °C. All the products were fully characterized by IR and NMR spectroscopy, and the molecular structures of [RuCl2(p-cymene)(SOC·IMes)] (3) and [RuCl2(p-cymene)(SOC·SIMes)] (5) were determined by X-ray diffraction analysis. Coordination of the NHC·COS ligands took place via the sulfur atom. A remarkable shielding of the methine proton on the p-cymene isopropyl group was observed by 1H NMR spectroscopy for complexes 3-6. It is most likely caused by the aromatic ring current of a neighboring mesityl or 2,6-diisopropylphenyl substituent. The catalytic activity of compounds 2-6 was probed in the ring-opening metathesis polymerization (ROMP) of cyclooctene, in the atom transfer radical polymerization (ATRP) of methyl methacrylate, and in the synthesis of enol esters from 1-hexyne and 4-acetoxybenzoic acid. In all these reactions, the [RuCl2(p-cymene)(SOC·NHC)] complexes displayed performances slightly inferior to those exhibited by [RuCl2(p-cymene)(NHC)] species that result from the reaction of [RuCl2(p-cymene)]2 with NHC·CO2 inner salts. However, they were significantly better catalyst precursors than the much more robust chelates of the [RuCl(p-cymene)(S2C·NHC)]PF6 type obtained by coordination of NHC·CS2 betaines to the ruthenium dimer. These results suggest that the Ru-(SOC·NHC) motif undergoes a dethiocarboxylation under the experimental conditions adopted for the catalytic tests and leads to the same elusive Ru-NHC active species as the preformed [RuCl2(p-cymene)(NHC)] family of complexes.
[Full Text] [<< Previous Article] [Back to the List of Publications] [Next Article >>] l.delaude@ulg.ac.be