"Synthesis and Organocatalytic Applications of Imidazol(in)ium-2-thiocarboxylates"
Hans Morgan, Johan Wouters, Albert Demonceau, and Lionel Delaude
 |
source: European Journal of Organic Chemistry
year: 2011
first page: 7083
last page: 7091
doi: 10.1002/ejoc.201101286
|
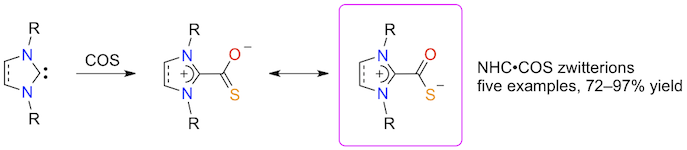
Abstract: Five imidazol(in)ium-2-thiocarboxylates bearing cyclohexyl, mesityl, or 2,6-diisopropylphenyl substituents on their nitrogen atoms were prepared from the corresponding imidazol(in)ium chlorides or tetrafluoroborates in a one-pot, two-step procedure involving the in situ generation of free N-heterocyclic carbenes (NHCs) with a strong base followed by trapping with carbonyl sulfide. The resulting NHC·COS zwitterions were isolated in high yields and characterized by IR and NMR spectroscopy. The molecular structure of SIMes·COS was determined by X-ray diffraction analysis. Experimental data and DFT calculations indicated that the negative charge on the thiocarboxylate anion is preferentially delocalized on the sulfur atom. Thermogravimetric analysis showed that the NHC·COS zwitterions undergo thermolysis at temperatures ranging between 110 and 180 °C in the solid state. They are also rather labile in solution. Unlike the related NHC·CS2 betaines, which are highly stable, crystalline materials, they displayed the same type of behavior as the analogous carboxylate adducts, which readily lose their CO2 moiety upon heating or dissolution. Thus, imidazol(in)ium-2-thiocarboxylates acted as convenient NHC precursors in two model organocatalytic transformations. Of the five thiocarboxylates examined, ICy·COS was the most efficient at promoting the acylation of benzyl alcohol with vinyl acetate, whereas SIMes·COS afforded the highest activity in benzoin condensation.
Keywords: Condensation Reactions, Carbenes, Organocatalysis, Transesterification, Zwitterions
[Full Text] [<< Previous Article] [Back to the List of Publications] [Next Article >>] l.delaude@ulg.ac.be