"An Unexpected Synthesis of Dihydrophenazines en Route to Benzimidazolium Salts"
Yannick Borguet, Guillermo Zaragoza, Albert Demonceau, and Lionel Delaude
 |
source: Advanced Synthesis & Catalysis
year: 2012
volume: 354
first page: 1356
last page: 1362
doi: 10.102/adsc.201100890
|
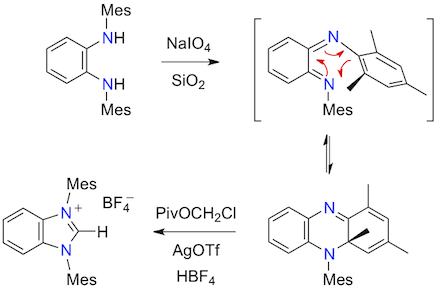
Abstract: The oxidation of various N,N'-diarylbenzene-1,2-diamines bearing bulky aromatic substituents with sodium periodate on wet silica gel afforded a series of five new dihydrophenazines instead of the expected cyclohexadiene-1,2-diimines. The reaction most likely proceeds via a 1,6-electrocyclic path and provides a convenient access to an important class of nitrogen heterocycles. Subsequent treatment of the mesityl derivative with chloromethyl pivalate and silver triflate led to the corresponding benzimidazolium salt.
Keywords: Electrocyclic Reactions, Ligand Design, Nitrogen Heterocycles, Oxidation, Sigmatropic Rearrangement
[Full Text] [<< Previous Article] [Back to the List of Publications] [Next Article >>] l.delaude@ulg.ac.be