"N-Heterocyclic Carbene Catalyzed Carba-, Sulfa-, and Phospha-Michael Additions with NHC·CO2 Adducts as Precatalysts"
Morgan Hans, Lionel Delaude, Jean Rodriguez, and Yoann Coquerel
 |
source: Journal of Organic Chemistry
year: 2014
volume: 79
first page: 2758
last page: 2764
doi: 10.1021/jo500108a
|
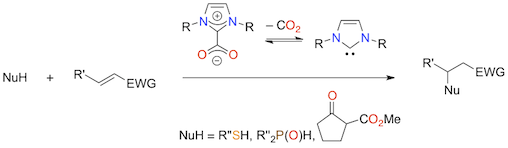
Abstract: N-heterocyclic carbene catalyzed Michael additions have been revisited with 1,3-dialkyl- or 1,3-diarylimidazol(in)ium-2-carboxylates, that is NHC·CO2 adducts, as the source of the free NHC catalysts in solution. Using these precatalysts, a number of efficient carba-, sulfa-, and phospha-Michael additions were achieved very conveniently, without the need for an external strong base to generate the NHC by deprotonation of an azolium salt. To further expand the scope of the procedure, some NHC-catalyzed sulfa-Michael/aldol organocascades were also investigated.
[Full Text] [<< Previous Article] [Back to the List of Publications] [Next Article >>] l.delaude@ulg.ac.be