"Ruthenium Catalysts Bearing a Benzimidazolylidene Ligand for the Metathetical Ring-Closure of Tetrasubstituted Cycloolefins"
Yannick Borguet, Guillermo Zaragoza, Albert Demonceau, and Lionel Delaude
 |
source: Dalton Transactions
year: 2015
volume: 44
first page: 9744
last page: 9755
doi: 10.1039/c5dt00433k
|
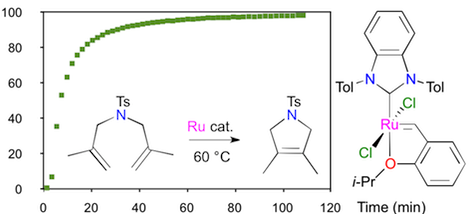
Abstract: Deprotonation of 1,3-di(2-tolyl)benzimidazolium tetrafluoroborate with a strong base afforded 1,3-di(2-tolyl)benzimidazol-2-ylidene (BTol), which dimerized progressively into the corresponding dibenzotetraazafulvalene. The complexes [RhCl(COD)(BTol)] (COD is 1,5-cyclooctadiene) and cis-[RhCl(CO)2(BTol)] were synthesized to probe the steric and electronic parameters of BTol. Comparison of the percentage of buried volume (%VBur) and of the Tolman electronic parameter (TEP) of BTol with those determined previously for 1,3-dimesityl¬benzimidazol-2-ylidene (BMes) revealed that the two N-heterocyclic carbenes displayed similar electron donicities, yet the 2-tolyl substituents took a slightly greater share of the rhodium coordination sphere than the mesityl groups, due to a more pronounced tilt. The anti,anti conformation adopted by BTol in the molecular structure of [RhCl(COD)(BTol)] ensured nonetheless a remarkably unhindered access to the metal center, as evidenced by steric maps. Second-generation ruthenium-benzylidene and isopropoxybenzylidene complexes featuring the BTol ligand were obtained via phosphine exchange from the first generation Grubbs and Hoveyda-Grubbs catalysts, respectively. The atropisomerism of the 2-tolyl substituents within [RuCl2(=CHPh)(PCy3)(BTol)] was investigated by using variable temperature NMR spectroscopy, and the molecular structures of all four possible rotamers of [RuCl2(=CH-o-OiPrC6H4)(BTol)] were determined by X-ray crystallography. Both complexes were highly active at promoting the ring-closing metathesis (RCM) of model α,ω-dienes. The replacement of BMes with BTol was particularly beneficial to achieve the ring-closure of tetrasubstituted cycloalkenes. More specifically, the stable isopropoxybenzylidene chelate enabled an almost quantitative RCM of two challenging substrates, viz. diethyl 2,2-bis(2-methylallyl)malonate and N,N-bis(2-methylallyl)tosylamide, within a few hours at 60 °C.
[Full Text] [<< Previous Article] [Back to the List of Publications] [Next Article >>] l.delaude@ulg.ac.be