"Probing the Diastereoselectivity of Staudinger Reactions Catalyzed by N-Heterocyclic Carbenes"
Hans Morgan, Johan Wouters, Albert Demonceau, and Lionel Delaude
 |
source: Chemistry - A European Journal
year: 2015
volume: 21
first page: 10870
last page: 10877
doi: 10.1002/chem.201501060
|
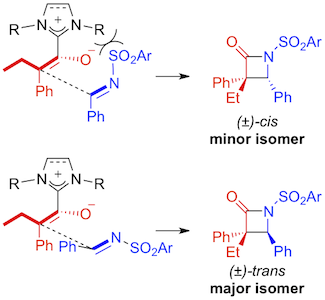
Abstract: The reaction of ethylphenylketene with 1,3-dimesitylimidazol-2-ylidene (IMes) or 1,3-dimesitylimidazolin-2-ylidene (SIMes) afforded the corresponding azolium enolates in high yields. The two zwitterions were fully characterized by various analytical techniques. Their thermal stabilities were monitored by TGA and the molecular structure of SIMes·EtPhC=C=O was determined by using X-ray crystallography. A mechanism was proposed to account for the trans-diastereoselectivity observed in the [2+2] cycloaddition of ketenes and N-protected imines catalyzed by N-heterocyclic carbenes and an extensive catalytic screening was performed to test its validity. The steric bulk of the NHC catalyst markedly affected the cis:trans ratio of the model β-lactam product. The nature of the solvent used to carry out the Staudinger reaction also significantly influenced its diastereoselectivity. Conversely, the nature of the substituent on the N-sulfonated imine reagent and the reaction temperature were less critical parameters.
Keywords: Carbenes, Lactams, Organocatalysis, Reaction Mechanisms, Zwitterions
[Full Text] [<< Previous Article] [Back to the List of Publications] [Next Article >>] l.delaude@ulg.ac.be