"Ruthenium-Arene Catalysts Bearing N-Heterocyclic Carbene Ligands for Olefin Cyclopropanation and Metathesis"
Michaël Méret, Anna M. Maj, Albert Demonceau, and Lionel Delaude
 |
source: Monatshefte für Chemie
year: 2015
volume: 146
first page: 1099
last page: 1105
doi: 10.1007/s00706-015-1492-x
|
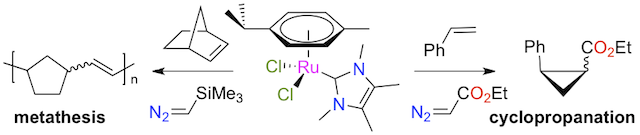
Abstract: Ruthenium-arene complexes bearing N-heterocyclic carbene (NHC) ligands with the generic formula [RuCl2(p-cymene)(NHC)] are efficient catalyst precursors for the cyclopropanation of activated olefins with ethyl diazoacetate, and the cis/trans diastereoselectivity of the reaction markedly depends on the steric bulk of the NHC. The procedure was successfully applied to styrene, α-methylstyrene, and various other styrenic derivatives bearing electron-withdrawing or donating substituents on their aromatic rings. The reaction of unactivated internal or terminal alkenes was more sluggish, and the use of norbornene as a substrate afforded only olefin metathesis. Further investigation of the ring-opening metathesis polymerization of this strained cycloolefin in the presence of trimethylsilyldiazomethane afforded high molecular weight polynorbornene whose microstructure was not significantly affected by the choice of the NHC ancillary ligand.
Keywords: Arene Complexes, Cross-Metathesis, para-Cymene, Homogeneous Catalysis, Ring-Opening Metathesis Polymerization, Structure-Activity Relationships
[Full Text] [<< Previous Article] [Back to the List of Publications] [Next Article >>] l.delaude@ulg.ac.be