"Synthesis, Characterization, and Catalytic Evaluation of Ruthenium-Diphosphine Complexes Bearing Xanthate Ligands"
Mohammed Zain Aldin, Anthony Maho, Guillermo Zaragoza, Albert Demonceau, and Lionel Delaude
 |
source: Dalton Transactions
year: 2018
volume: 47
first page: 13926
last page: 13938
doi: 10.1039/c8dt02838a
|
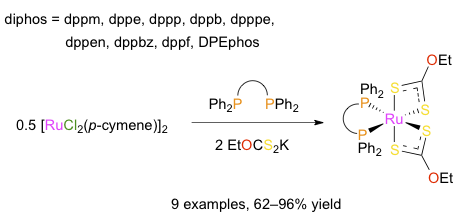
Abstract: The reaction of [RuCl2(p-cymene)]2 with potassium O-ethylxanthate and a set of nine representative Ph2P-X-PPh2 bidentate phosphines (dppm, dppe, dppp, dppb, dpppe, dppen, dppbz, dppf, and DPEphos) afforded monometallic [Ru(S2COEt)2(diphos)] chelates 1-9 in 62-96% yield. All the products were fully characterized by using various analytical techniques and their molecular structures were determined by X-ray crystallography. They featured a highly distorted octahedral geometry with a S-Ru-S bite angle close to 72° and P-Ru-P angles ranging between 73° and 103°. Bond lengths and IR stretching frequencies recorded for the anionic xanthate ligands strongly suggested a significant contribution of the EtO+=CS22- resonance form. 1H NMR and XRD analyses showed that the methylene protons of the ethyl groups were diastereotopic due to a strong locking of their conformation by a neighboring phenyl ring. On cyclic voltammetry, quasi-reversible waves were observed for the Ru2+/Ru3+ redox couples with E1/2 values comprised between 0.65 and 0.80 V vs. Ag/AgCl. The activity of chelates 1-9 was probed in three catalytic processes, viz., the synthesis of vinyl esters from benzoic acid and 1-hexyne, the cyclopropanation of styrene with ethyl diazoacetate, and the atom transfer radical addition of carbon tetrachloride and methyl methacrylate. In the first case, 31P NMR analysis of the reaction mixtures showed that the starting complexes remained mostly unaltered despite the harsh thermal treatment that was applied to them. In the second case, monitoring the rate of nitrogen evolution revealed that all the catalysts under investigation behaved similarly and were rather slow initiators. In the third case, [Ru(S2COEt)2(dppm)] was singled out as a very active and selective catalyst already at 140 °C, whereas most of the other complexes resisted degradation up to 160 °C and were only moderately active. Altogether, these results were in line with the high stability displayed by [Ru(S2COEt)2(diphos)] chelates 1-9.
[Full Text] [<< Previous Article] [Back to the List of Publications] [Next Article >>] l.delaude@ulg.ac.be