"Rhodium Catalysts with Superbulky NHC Ligands for the Selective α-Hydrothiolation of Alkynes"
Malgorzata Bolt, Lionel Delaude, and Patrycja Zak
 |
source: Dalton Transactions
year: 2022
volume: 51
first page: 4429
last page: 4432
doi: 10.1039/d2dt00243d
|
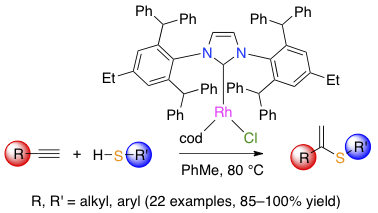
Abstract: Eight rhodium complexes — including four new compounds — with the generic formula [RhCl(cod)(NHC)] (cod is 1,3-cyclooctadiene) differing by the size of their N-heterocyclic carbene (NHC) ligand were prepared, characterized, and found to be catalytically active in the hydrothiolation of terminal alkynes with aliphatic or aromatic thiols. The steric bulk of the carbene was found to markedly influence the reaction rate and selectivity. In particular, superbulky NHCs led to the almost quantitative formation of the sole α-vinyl sulfide products. The experimental conditions were optimized to allow the straightforward synthesis of a broad range of mono- and disubstituted α-adducts starting from terminal alkynes (18 examples) and thiols (5 examples). Altogether, the procedure devised in this study provides an easy access to α-vinyl sulfides with full atom economy and a low catalyst loading.
[Full Text] [<< Previous Article] [Back to the List of Publications] [Next Article >>] l.delaude@ulg.ac.be