"Caffeine and Theophylline as Sustainable, Biosourced NHC Ligand Precursors for Efficient Palladium-Catalyzed Suzuki-Miyaura Cross-Coupling Reactions"
François Mazars, Guillermo Zaragoza, and Lionel Delaude
 |
source: Journal of Organometallic Chemistry
year: 2022
volume: 978
article: 122489
pages: 11
doi: 10.1016/j.jorganchem.2022.122489
|
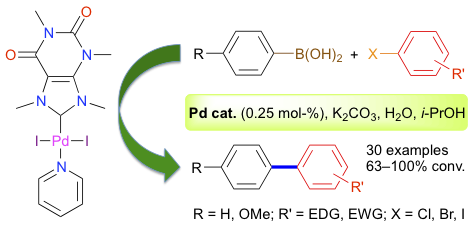
Abstract: The alkylation of caffeine with methyl iodide afforded 1,3,7,9-tetramethylxanthinium iodide, which was further reacted with PdCl2, KI, and K2CO3 in neat pyridine to afford the [PdI2(NHC)(Py)] complex 1 in two steps and 73% overall yield. An intermediate anion exchange afforded the corresponding dichlorido complex 2 in 70% overall yield. Theophylline was successfully converted into a new xanthinium salt bearing an isobutyl group on its N7 atom, which was further employed to prepare the [PdCl2(NHC)(Py)] complex 3. These three Pd-PEPPSI catalyst precursors were fully characterized and the molecular structure of complex 2 was determined. Complexes 1-3 displayed a high catalytic activity in the Suzuki-Miyaura cross-coupling of aryl halides with phenylboronic acids. Aryl bromides and iodides bearing either electron-donating or withdrawing substituents afforded a wide range of biaryl derivatives that were isolated in 40-93% yields (30 examples). The reactions required only a low catalyst loading (0.25 mol-%) and were carried out in a green, water-based solvent mixture using K2CO3 as a base in the presence of air. Although less reactive, aryl chlorides were also successfully activated under more forcing conditions, provided that an electron-withdrawing group was present on their aromatic ring.
Keywords: Alkaloids, Biaryls, Carbene Ligands, Green Chemistry, Palladium
[Full Text] [<< Previous Article] [Back to the List of Publications] [Next Article >>] l.delaude@ulg.ac.be