"The Facile Hydrolysis of Imidazolinium Chlorides (N-Heterocyclic Carbene Precursors) Under Basic Aqueous Conditions"
Nedra Touj, Jerwin Jay Taping, Nikolay Tumanov, Johan Wouters, and Lionel Delaude
 |
source: Chemistry - A European Journal
year: 2023
volume: 29
article: e202302402
pages: 10
doi: 10.1002/chem.202302402
|
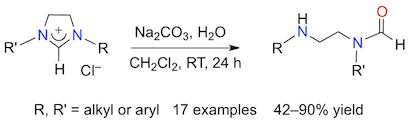
Abstract: The hydrolysis of imidazolinium chlorides takes place readily in a basic water/dichloromethane biphasic mixture at room temperature. Due to their aromatic nature, imidazolium and benzimidazolium salts are much more resistant to ring-opening under these conditions. Experimental parameters were optimized to afford a full conversion and high yields of γ-aminoformamides starting from twelve symmetrical substrates with alkyl or aryl substituents on their nitrogen atoms, and five unsymmetrical 1-alkyl-3-arylimidazolinium chlorides. NMR and XRD analyses showed that the cleavage of unsymmetrical salts led to γ-alkylamino-N-arylformamides with a high regioselectivity and that bulky alkyl or aryl groups on the formamide moiety led to the isolation of the (E)-isomer in high stereoisomeric purity (>95%), whereas smaller and more flexible alkyl substituents afforded mixtures of (E)- and (Z)-rotamers. Control experiments showed that the hydrolysis of 1,3-dimesitylimidazolinium chloride (SIMes·HCl) did not occur readily in pure or acidic water and that the presence of bulky aromatic substituents on the nitrogen atoms of 1,3-bis(2,6-diisopropylphenyl)imidazolinium chloride (SIDip·HCl) efficiently slowed down its hydrolysis under basic aqueous conditions. Most strikingly, this work highlighted the critical influence of the counter-anion on the reactivity of imidazolinium cations. Indeed, the chloride salts underwent a facile hydrolysis in the presence of water and Na2CO3, whereas various other NHC·HX derivatives reacted much slower or remained essentially inert under these conditions.
Keywords: Amides, Amines, Carbene Ligands, Hydrolysis, Synthetic Methods
[Full Text] [<< Previous Article] [Back to the List of Publications] [Next Article >>] l.delaude@ulg.ac.be