"Synthesis, Characterization, and Catalytic Evaluation of Ruthenium Complexes Bearing Xanthinium-8-dithiocarboxylate Ligands Derived from Caffeine and Theophylline"
François Mazars, Guillermo Zaragoza, and Lionel Delaude
 |
source: Organometallics
year: 2024
volume: 43
first page: 2284
last page: 2304
doi: 10.1021/acs.organomet.4c00315
|
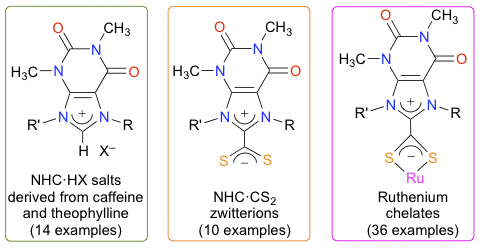
Abstract: Various experimental procedures and purification techniques were applied to alkylate or arylate the N7 and N9 positions of caffeine and theophylline into xanthinium salts. These N-heterocyclic carbene (NHC) precursors were converted into xanthinium-8-dithiocarboxylate zwitterions using CS2 and either Cs2CO3 or NaOtBu. The NHC·CS2 betaines were employed as chelating ligands to prepare a wide variety of [RuX(p-cymene)(S2C·NHC)]Y (X = Cl, SAc; Y = Cl, PF6, [RuCl3(p-cymene)]) and [Ru(S2C ·NHC)3]X2 (X = Cl, PF6) complexes that were characterized by NMR and HRMS. Moreover, the molecular structures of three betaines, one hetero-, and one homoleptic complex were determined by XRD. The catalytic potentials of all these complexes were investigated in the transfer hydrogenation of ketones with isopropanol, the synthesis of vinyl esters from benzoic acid and 1-hexyne, and the cyclopropanation of styrene with ethyl diazoacetate. The reduction of acetophenone into 1-phenylethanol was chosen as a model reaction for the former application. Monitoring the time course of this transformation showed that chelates bearing a NHC·CS2 ligand displayed an initial activity slightly higher than the analogous [RuCl2(p-cymene)(NHC)] complex. Contrastingly, for the last two catalytic processes, the Ru(S2C·NHC) chelates did not outperform their Ru-NHC counterparts.
[Full Text] [<< Previous Article] [Back to the List of Publications] [Next Article >>] l.delaude@ulg.ac.be