"Facile and Regioselective Deuteration of C2-Alkylated Imidazolium salts in the Presence of Cesium Carbonate"
François Mazars, Johann Far, Christian Damblon, and Lionel Delaude
 |
source: Chemistry - A European Journal
year: 2025
volume: 31
article: e202404315
pages: 7
doi: 10.1002/chem.202404315
|
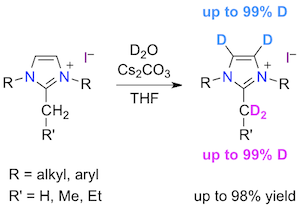
Abstract: Thirteen imidazolium iodides bearing benzyl, mesityl, or 2,6-diisopropylphenyl substituents on their nitrogen atoms, and C1 to C4 alkyl chains on their C2 carbon atom were readily deuterated with D2O as a cheap and non-toxic deuterium source in the presence of Cs2CO3, a weak, innocuous, inorganic base. The isotopic exchange proceeded quickly and efficiently under mild, aerobic conditions to afford a range of aNHC and NHO precursors regioselectively labeled on their C2α exocyclic position and/or C4=C5 heterocyclic backbone. A “carbene-free” mechanism was postulated, in which the carbonate anion acts as a catalyst to activate an exocyclic, acidic C-H bond and ease a deuterium transfer from D2O to the imidazolium salt in a concerted fashion.
Keywords: Carbenes, Deuterium; Ionic Liquids; Nitrogen Heterocycles; Regioselectivity
[Full Text] [<< Previous Article] [Back to the List of Publications] [Next Article >>] l.delaude@ulg.ac.be