"Mechanistic Insight into the Staudinger Reaction Catalyzed by N-Heterocyclic Carbenes"
Hans Morgan, Johan Wouters, Albert Demonceau, and Lionel Delaude
 |
source: Chemistry - A European Journal
year: 2013
volume: 19
first page: 9668
last page: 9676
doi: 10.1002/chem.201204428
|
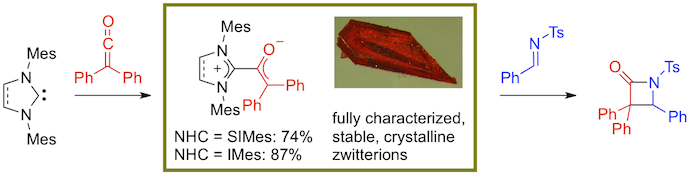
Abstract: Four zwitterions were prepared by reacting 1,3-dimesitylimidazolin-2-ylidene (SIMes) or 1,3-dimesitylimidazol-2-ylidene (IMes) with either N-tosyl benzaldimine or diphenylketene. They were isolated in high yields and characterized by IR and NMR spectroscopy. The molecular structures of three of them were determined by using X-ray crystallography and their thermal stability was monitored by using thermogravimetric analysis. The imidazol(in)ium-2-amides were rather labile white solids that did not show any tendency to tautomerize into the corresponding 1,2,2-triaminoethene derivatives. They displayed a mediocre catalytic activity in the Staudinger reaction of N-tosyl benzaldimine with diphenylketene. In contrast, the imidazol(in)ium-2-enolates were orange-red crystalline materials that remained stable over extended periods of time. Despite their greater stability, these zwitterions turned out to be efficient promoters for the model cycloaddition under scrutiny. As a matter of fact, their catalytic activity matched those recorded with the free carbenes. Altogether, these results provide strong experimental insight into the mechanism of the Staudinger reaction catalyzed by N-heterocyclic carbenes. They also highlight the superior catalytic activity of the imidazole-based carbene IMes compared to its saturated analogue SIMes in the reaction under consideration.
Keywords: Carbenes, Lactams, Organocatalysis, Reaction Mechanisms, Zwitterions
[Full Text] [<< Previous Article] [Back to the List of Publications] [Next Article >>] l.delaude@ulg.ac.be